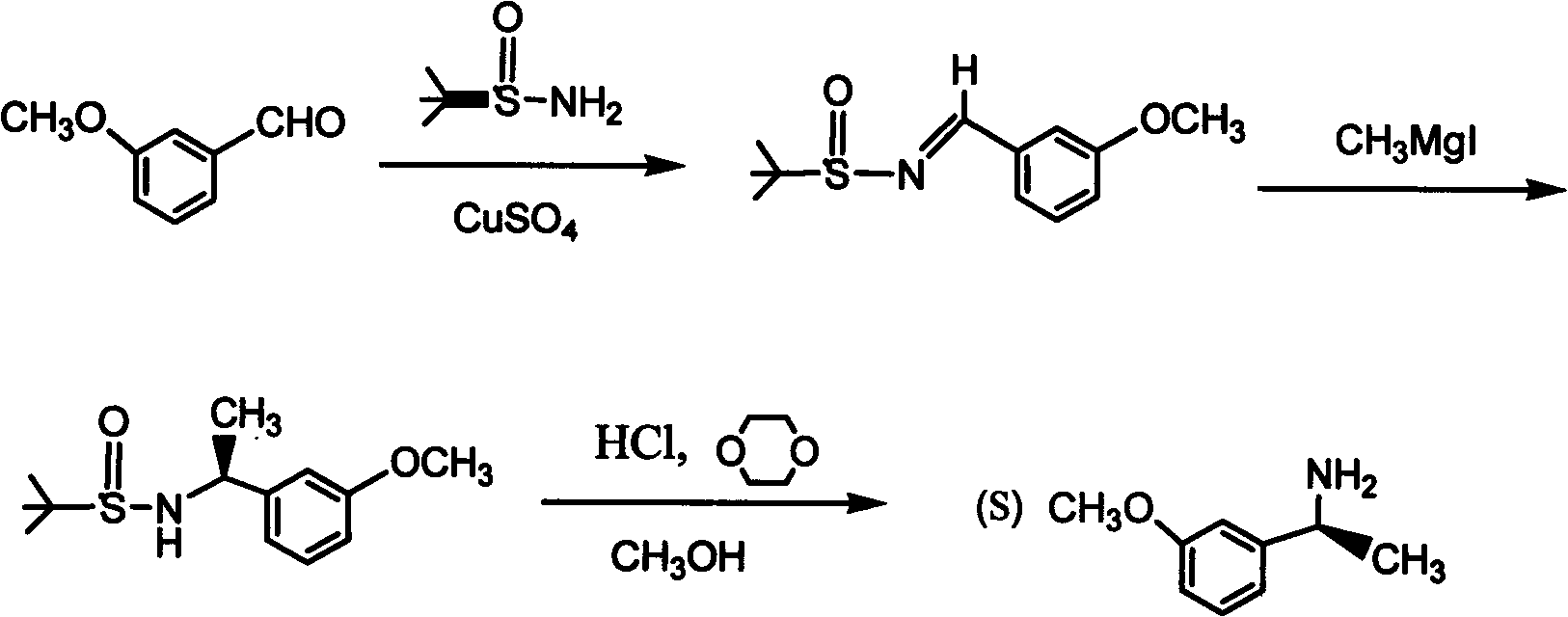
(S)-3-(1-dimethylaminoethyl)phenol preparation method
A technology of dimethylaminoethyl and dimethylbenzylamine, which is applied in the field of drug synthesis, can solve the problems of non-reuse of R-enantiomer, large loss of raw materials, and high production cost, so as to achieve cheap raw materials and increase yield , cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of (S)-m-benzyloxy-α,N-dimethylbenzylamine (III)
[0041] 30.7g of L-tartaric acid, 49.3g of (RS) m-benzyloxy-α,N-dimethylbenzylamine, 320ml of ethanol, stirring and refluxing for 1 hour, cooling to room temperature, standing overnight, precipitation of crystals, and filtration to obtain a crude product. Recrystallize from ethanol to obtain 24.2g of salt, add 100ml of water, adjust pH>10 with 30% NaOH solution, extract with toluene, dry with anhydrous sodium sulfate and evaporate the solvent to obtain 14.9g of residual liquid [α] D -42° (C, 5, MeOH), optical purity>99% e.e., 60.5% of theoretical yield.
[0042] 1 HNMR(CD 3 OD): δ 1.27 (d, 3H), 2.12 (s, 3H), 3.52 (q, 1H), 5.00 (s, 2H), 6.79-6.83 (m, 2H), 6.90-6.91 (t, 1H) ,7.14-7.39 (m, 6H).
Embodiment 2
[0043] Example 2 Resolution of mother liquor, recovery and racemization to prepare (RS) m-benzyloxy-α,N-dimethylbenzylamine (II)
[0044] Example 1 After the resolution of the mother liquor, after recovering ethanol under normal pressure, add 30% NaOH solution to make the pH>10, extract with petroleum ether or ethyl acetate, wash with saturated NaCl solution, anhydrous Na 2 SO 4 After drying, the solvent was recovered to obtain 31 g of residual liquid. Add 24ml of DMSO or toluene, 2.2g of potassium tert-butoxide, and react at 100°C for 2.5 hours. Recover the solvent, extract with petroleum ether or ethyl acetate, wash with saturated NaCl solution, dry with anhydrous sodium sulfate, and evaporate the solvent to obtain (RS) m-benzyloxy-α,N-dimethylbenzylamine 30.2g, [α] D0.00-0.03° (C, 5, MeOH), the recovery rate is 96.8%, the content is 98.6% (GC), which can be used for resolution.
Embodiment 3
[0045] Example 3 Preparation of (S)-3-(1-dimethylaminoethyl)phenol (I)
[0046] (S)-m-benzyloxy-α,N-dimethylbenzylamine 35g, 38% formaldehyde solution 18.34g, methanol 630ml, Reynolds nickel 17.5ml, hydrogenation at room temperature and atmospheric pressure for amine methylation, while hydrogen The benzyl group is decomposed to obtain 23.5g of product, the yield is 98%, recrystallized from ethanol, mp118-119℃, [α] D -55°(C, 5, EtOH), optical purity>99% e.e.
[0047] 1 HNMR(CDCl 3 ): δ 1.39(d, 3H), 2.23(s, 6H), 3.31(q, 1H), 6.72(d, 1H), 6.77(s, 1H), 6.78(d, 1H), 7.12-7.15( t, 1H), 7.5(br, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com