Amino aryloxy rare earth metal amide and application thereof
A technology of aminoaryloxy rare earth and amides, which is applied to lithium organic compounds, compounds of group 4/14 elements of the periodic table, compounds containing elements of group 3/13 of the periodic table, etc., to achieve the effect of simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
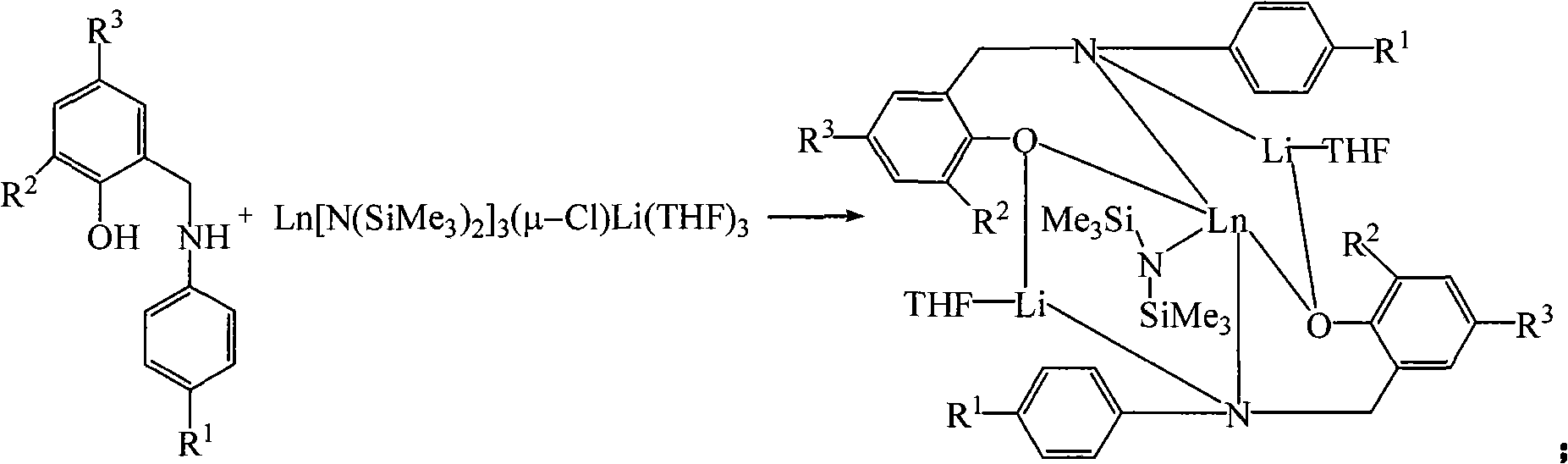
[0038] Example 1: Preparation of [NO] 2 NdN(SiMe 3 ) 2 Li 2 (THF) 2 , [NO]=p-R 1 -C 6 H 4 NCH 2 (3-R 2 -5-R 3 -C 6 H 2 -2-O), R 1 is methyl, R 2 , R 3 Both are tert-butyl groups.
[0039] (1) 1.61 g of [NO]H 2 (4.95 mmol) was dissolved in tetrahydrofuran, added with Nd[N(SiMe 3 ) 2 ] 3 (μ-Cl)Li(THF) 3 (4.95 mmol) in tetrahydrofuran solution, the system was blue, and the reaction was stirred at 25°C overnight;
[0040] (2) Remove the solvent, add 30 ml of toluene for heating extraction, centrifuge, transfer the clear liquid, add 4.00 ml of hexane, concentrate the solution to 20 ml, overnight at room temperature, 2.22 g (2.00 mmol) of blue crystals are precipitated, and the yield is 41 %.
Embodiment 2
[0041] Example 2: Preparation of [NO] 2 YbN(SiMe 3 ) 2 Li 2 (THF) 2 , [NO]=p-R 1 -C 6 H 4 NCH 2 (3-R 2 -5-R 3 -C 6 H 2 -2-O), R 1 is methyl, R 2 , R 3 Both are tert-butyl groups.
[0042] (1) will contain 0.72 g [NO]H 2 (2.21 mmol) in tetrahydrofuran was added to a solution containing 2.21 mmol of Yb[N(SiMe 3 ) 2 ] 3 (μ-Cl)Li(THF) 3 In the tetrahydrofuran solution, the system was yellow, and the reaction was stirred at 25 °C overnight;
[0043] (2) The tetrahydrofuran solvent was removed, 20 ml of toluene was added for heating extraction, centrifugation, and the clear liquid was transferred, 3.00 ml of hexane was added, the solution was concentrated to 20 ml, and 1.04 g (0.91 mmol) of bright yellow crystals were precipitated at room temperature overnight. The yield was was 41%.
Embodiment 3
[0044] Example three, preparation of [NO] 2 SmN(SiMe 3 ) 2 Li 2 (THF) 2 , [NO]=p-R 1 -C 6 H 4 NCH 2 (3-R 2 -5-R 3 -C 6 H 2 -2-O), R 1 is methyl, R 2 , R 3 Both are tert-butyl groups.
[0045] (1) will contain 1.49 g [NO]H 2 (4.58 mmol) in tetrahydrofuran was added to a solution containing 4.58 mmol of Sm[N(SiMe 3 ) 2 ] 3 (μ-Cl)Li(THF) 3 In the tetrahydrofuran solution, the system was yellow, and the reaction was stirred at 25 °C overnight;
[0046] (2) The tetrahydrofuran solvent was removed, 40 ml of toluene was added for heating extraction, centrifugation, and the clear liquid was transferred, 5.00 ml of hexane was added, the solution was concentrated to 30 ml, and a large amount of yellow crystals, 2.38 g (2.14 mmol), were precipitated at room temperature. The yield was 46%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com