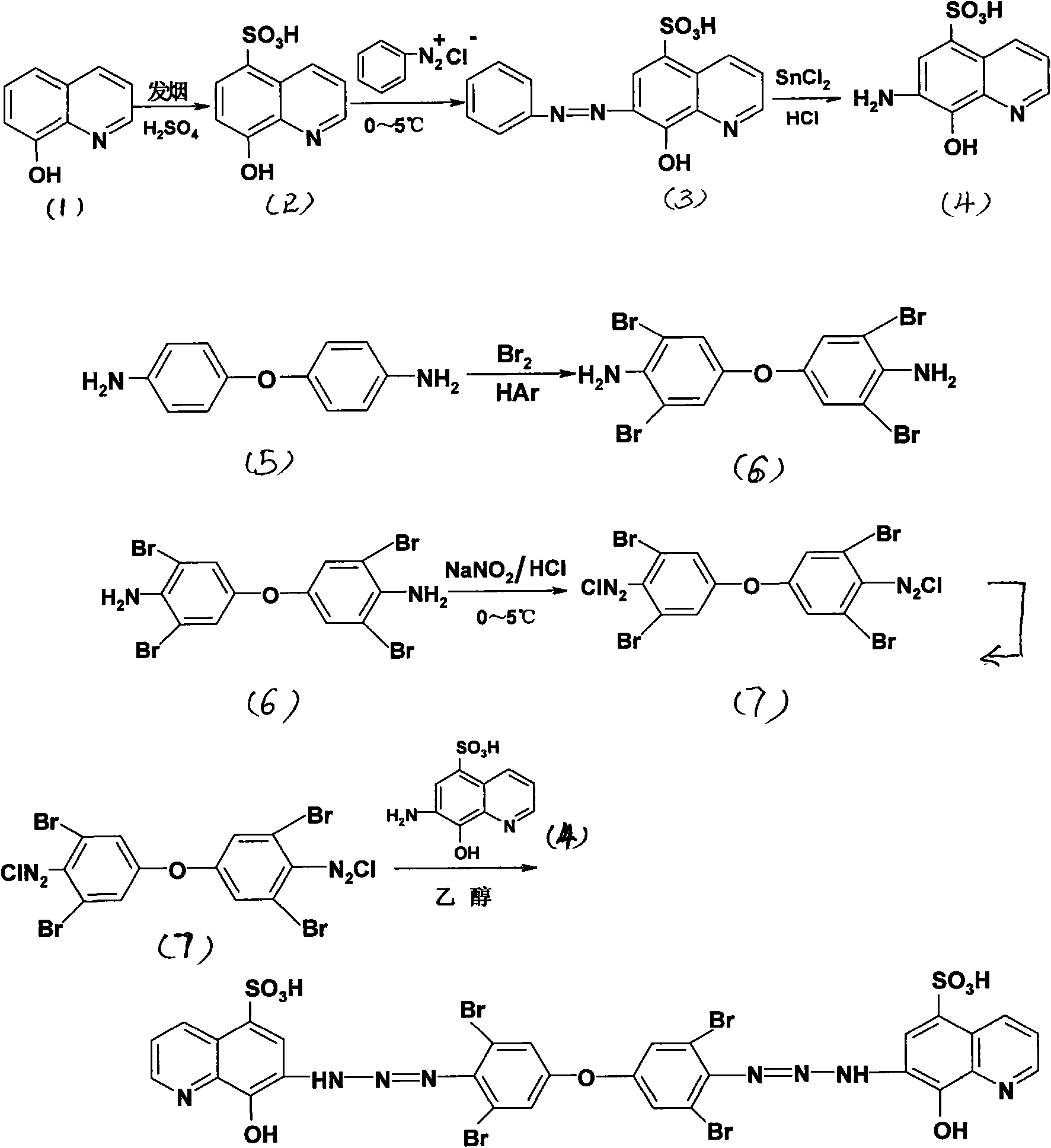
4,4'-bis(5-sulfo-8-hydroxyl-7-aminoquinoline azo)-3,5,3',5'-tetrabromo-diphenyl ether
A kind of technology of quinoline aminoazo group and tetrabromodiphenyl ether is applied in 4 fields, can solve the problems of few, coexisting ion interference and the like, and achieves the effects of low cost, high sensitivity and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 4,4'-bis(5-sulfonic acid-8-hydroxy-7-quinolineaminoazo)-3,5,3',5'-tetrabromodiphenyl ether (BSHQAABBE), its molecular formula:
[0033]
[0034] The preparation method of 4,4'-bis(5-sulfonic acid group-8-hydroxyl-7-quinoline aminoazo)-3,5,3',5'-tetrabromodiphenyl ether comprises the following steps:
[0035] 1, the preparation of 5-sulfonic acid group-8-hydroxyquinoline
[0036] Slowly dissolve 7.25g (0.05mol) of 8-hydroxyquinoline (1) in 50g of fuming sulfuric acid (containing 4% SO 3 ) at 8°C for 24 hours, pour it into 800g of crushed ice to obtain a large amount of precipitate, filter it, wash it thoroughly with cold water, and recrystallize it with about 5% to 10% dilute hydrochloric acid to obtain a colorless needle-like crystal 5 -Sulfonic acid-8-hydroxyquinoline (2), melting point 322-323°C.
[0037] 2, Preparation of 5-sulfonic acid group-7-azobenzene-8-hydroxyquinoline
[0038] Dissolve 3.72g (0.04mol) of aniline in 30mL of dilute hydrochloric acid (1:1), c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com