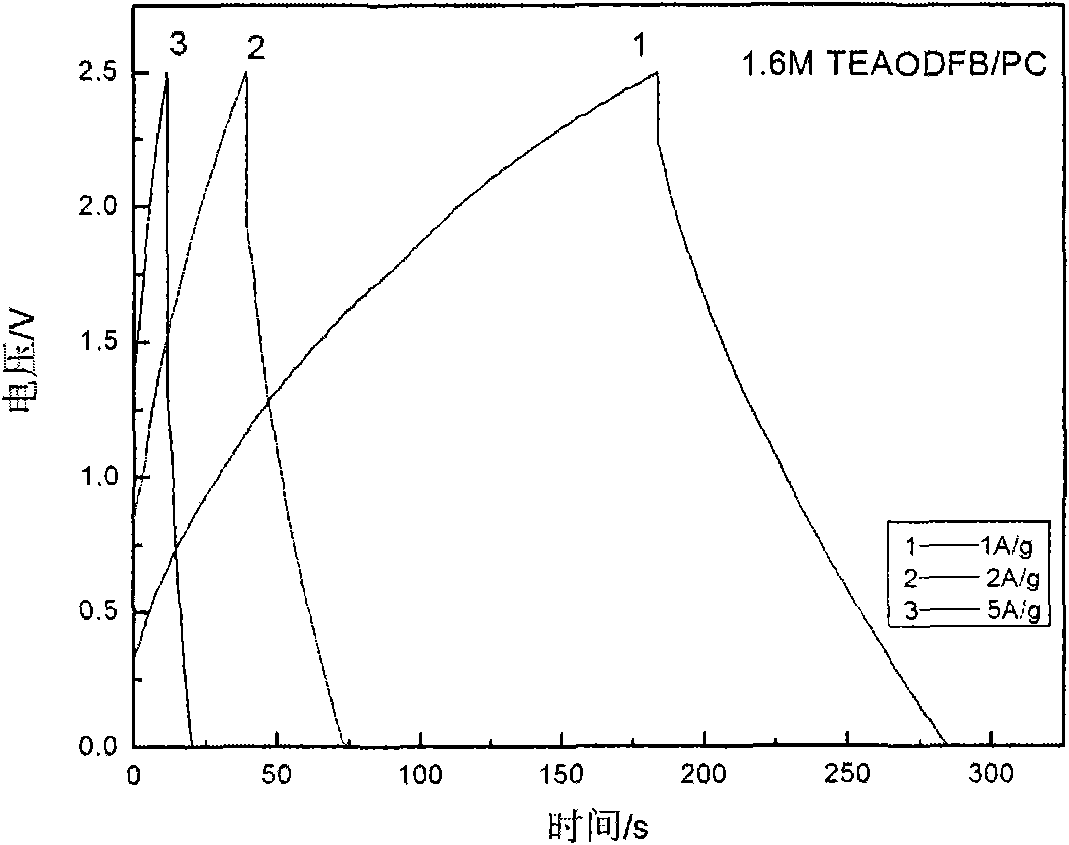
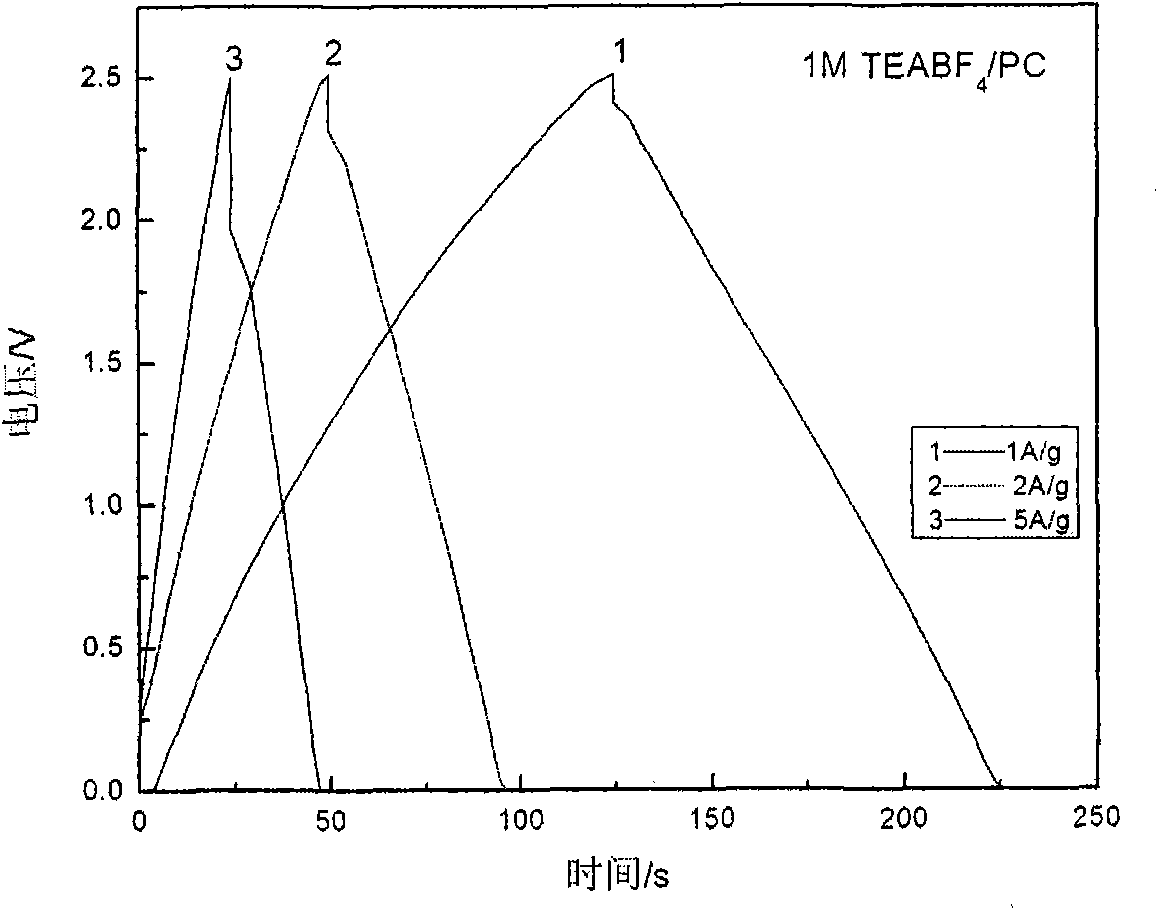
Low-temperature electrolyte for supercapacitor and preparation method thereof
A technology for electrolyte preparation and supercapacitors, applied in capacitors, electrolytic capacitors, circuits, etc., can solve problems such as low solubility, space constraints on energy density of supercapacitors, toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Using SiCl 4 , anhydrous oxalic acid, etc. to prepare TEAODFB in acetonitrile solution.
[0026] Step 1 Take 30.29g TEABF with an electronic balance in the glove box 4 , 12.55g of anhydrous oxalic acid was charged into a 500mL three-necked flask. Weigh 8mL SiCl with a 10mL graduated cylinder 4 Fill into a dropper bottle. TEABF 4 and anhydrous oxalic acid dissolved in 300mL acetonitrile, SiCl 4 Add dropwise during electromagnetic stirring. The reaction process was protected by high-purity argon at 20°C. SiCl 4 Adding is completed in about 6 hours, and then stirred for 10 hours after adding, the sign of the end of the reaction is that no gas is produced, and it is checked with pH test paper.
[0027] Step 2 Use a rotary evaporator to evaporate and concentrate the reacted solution at 35° C. until no liquid vapor is generated to obtain 27.7 g of the product.
[0028] Detection method: detect product purity with ICP, record the purity of product and be 99.28%, test ...
Embodiment 2
[0031] Using SiCl 4 , anhydrous oxalic acid, etc. to prepare TEAODFB in acetonitrile solution.
[0032] The steps are the same as in Example 1.
[0033] Step 2: Methyl formate was added according to the molar ratio of solvent and crystallizer of 12:1, the mixed solvent was recrystallized at -20°C for several times at low temperature, and 26.8 g of white crystals were obtained by filtration.
[0034] With example one, gained product is analyzed, and the result shows: the purity of product is 99.3%, moisture content (in H 2 O meter) is 8ppm.
Embodiment 3
[0036] Using SiCl 4 , anhydrous oxalic acid, etc. to prepare TEAODFB in PC+DMC solvent.
[0037] Step 1 Take 30.29g TEABF with an electronic balance in the glove box 4 , 12.55g of anhydrous oxalic acid was charged into a 500mL three-necked flask. Weigh 8mL SiCl with a 10mL graduated cylinder 4 Fill into a dropper bottle. TEABF 4 and anhydrous oxalic acid dissolved in 300mL PC+DMC (volume ratio 5:1) solution, SiCl4 Add dropwise during electromagnetic stirring. The reaction process was protected by high-purity argon at 10°C. SiCl 4 Adding is completed in about 6 hours, and then stirred for 10 hours after adding, the sign of the end of the reaction is that no gas is produced, and it is checked with pH test paper.
[0038] Step 2 Add methanol according to the molar ratio of solvent and crystallizer at 10:1, carry out multiple low-temperature recrystallizations on the mixed solvent at -30°C, and filter to obtain 26.2 g of white crystals.
[0039] With example one, gained pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com