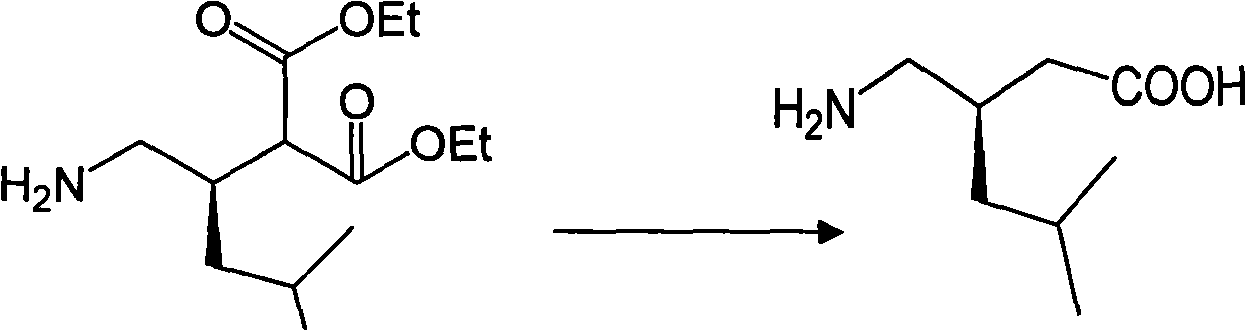Lyrica preparation method
A technology of pregabalin and diethyl isobutylmalonate, which is applied in the field of preparation of pregabalin, can solve the problems of unfavorable product industrialization, low yield, high toxicity, etc., and achieve easy quality control and by-product The effect of fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
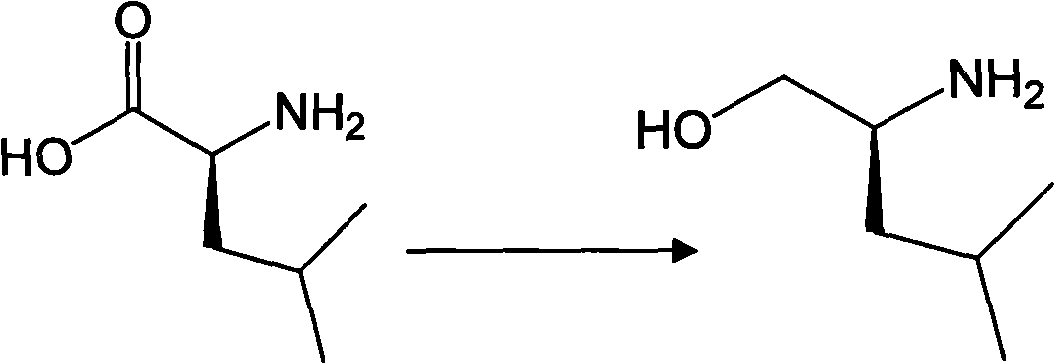
Embodiment 1
[0055] The preparation of embodiment 1 S-2-methyl-4-aminopentanol (formula viii)
[0056] Add 100ml of anhydrous tetrahydrofuran and 45.8g (0.35mol) of S-(+)-leucine into a 500ml three-neck flask, cool to 0°C in an ice bath under stirring, and slowly add 3.3g (0.087mol) of LiAlH 4 The mixture with 100ml of tetrahydrofuran was added dropwise, and the mixture was incubated and stirred for 3 hours. After the reaction is completed, dilute sulfuric acid is added dropwise to the reaction solution to adjust the pH value to neutral, then 300ml of water is added, extracted with 200ml×3 dichloromethane, the organic layer is separated, the organic layer is dried over anhydrous magnesium sulfate, and the organic layer is collected after filtration. layer, the organic solvent was recovered under reduced pressure, and the residue was subjected to column chromatography (petroleum ether: ethyl acetate = 20:1 v / v) to obtain 34.7 g of formula viii.
[0057] 1 H-NMR (CDCl 3 )
[0058] δ: 0.8...
Embodiment 2
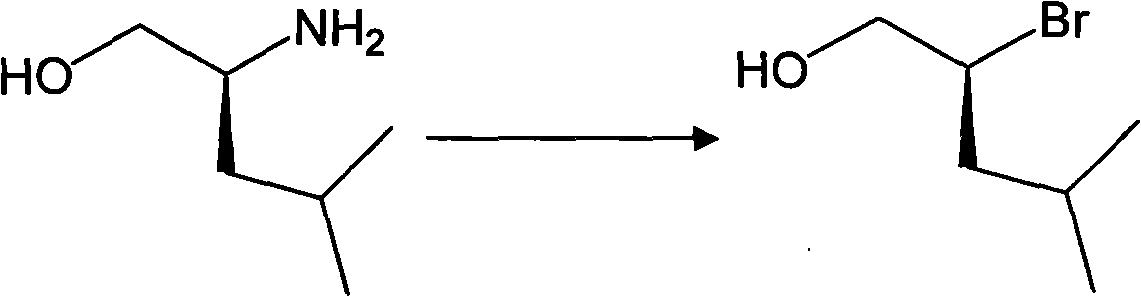
[0059] The preparation of embodiment 2 S-2-bromo-4-methylpentanol (formula vii)
[0060] Dissolve 11.7g (0.1mol) of S-2-methyl-4-aminopentanol and 46.1g (0.32mol) of cuprous bromide in 80ml of water, and slowly add 24.6ml of 48% hydrogen bromide dropwise under stirring at room temperature ( 0.22 mol), the dropwise addition was completed, the reaction system was cooled to -15°C, and 8.56 g (0.12 mol) of sodium nitrite was slowly added to the reaction system in batches under nitrogen protection (about 5 g / 15 minutes). After the addition was complete, the temperature was maintained and the reaction was stirred for 2.5 hours. Then the temperature was raised to 0° C. and the reaction was stirred for 6 hours. After the reaction was complete, 100 ml of ethyl acetate × 6 was used to extract the combined organic phases. The organic layer was dried over anhydrous magnesium sulfate, filtered, and the organic solvent was recovered under reduced pressure. The residue was subjected to colum...
Embodiment 3
[0061] Example 3 Preparation of S-2-bromo-4-methylpentyloxy-tert-butyldimethylsilane (formula vi)
[0062] Under nitrogen protection, 9.8g (54mmol) of S-2-bromo-4-methylpentanol, 0.66g (5.4mmol) of dimethylaminopyridine, 20ml of triethylamine and 175ml of dichloromethane were added to a 500ml three-necked flask. Cool to 0° C. in an ice bath, and slowly add 10.24 g (68 mmol) of tert-butyldimethylsilyl chloride. After the addition was complete, the temperature was maintained and the reaction was stirred for 30 minutes, and then stirred and reacted at room temperature for 5 hours. Add 200ml of distilled water and stir for 30 minutes, extract with 100ml×3 dichloromethane, combine the organic phase, and use 100ml of saturated NaHCO for the organic phase 3 Extraction and washing, and extraction and washing with distilled water three times, the organic layer was dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure, and the residue was subjected to co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com