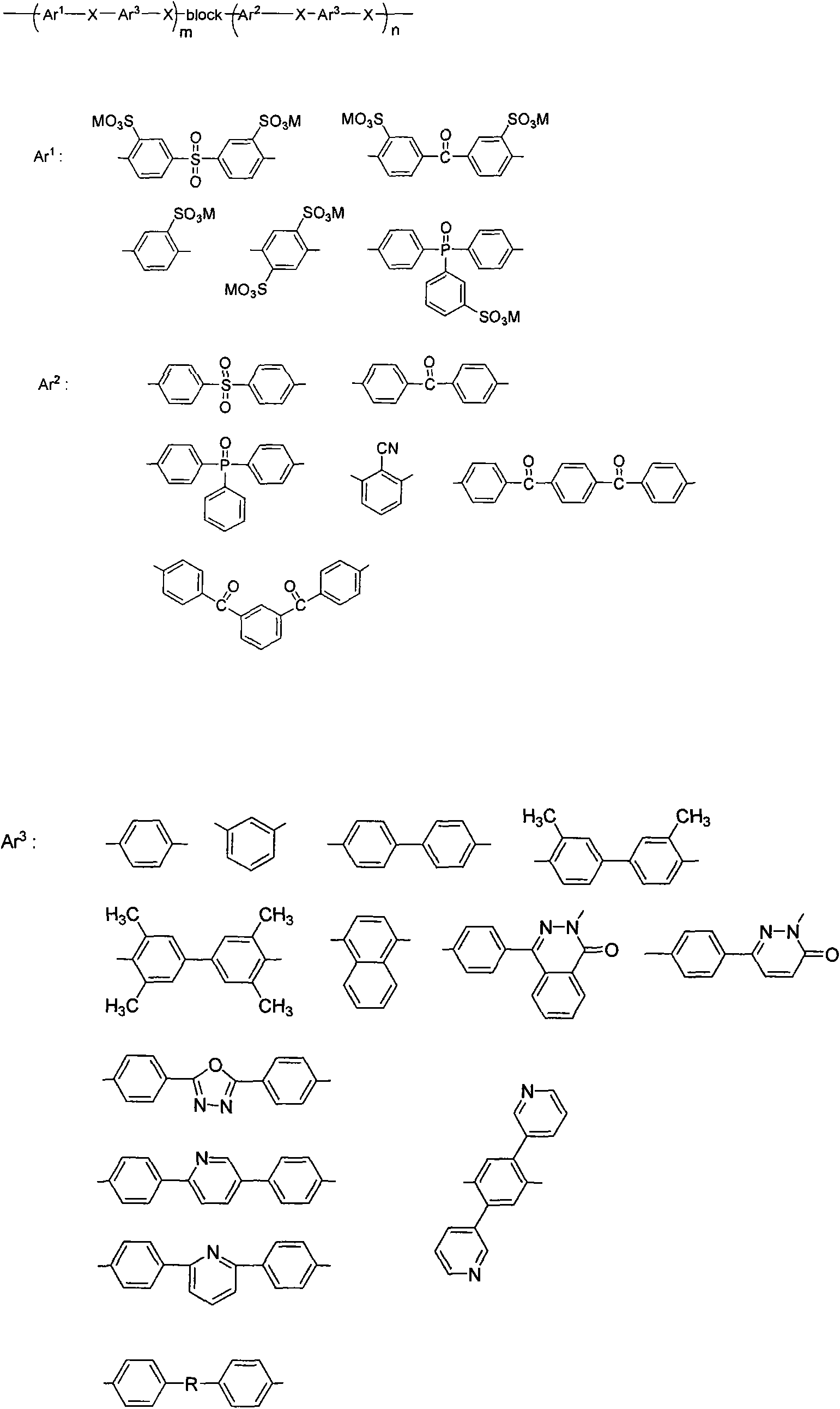
Efficient preparation method of block sulfonated aromatic poly (thio) ether
A technology of sulfonated polyarylamide and aromatic ether, which is applied in the field of high-efficiency preparation of novel block sulfonated polyaryl ether, can solve the problems of complicated recycling process, large reaction liquid volume, and long reaction time, and achieve simplified equipment composition and simplified process , the effect of high molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
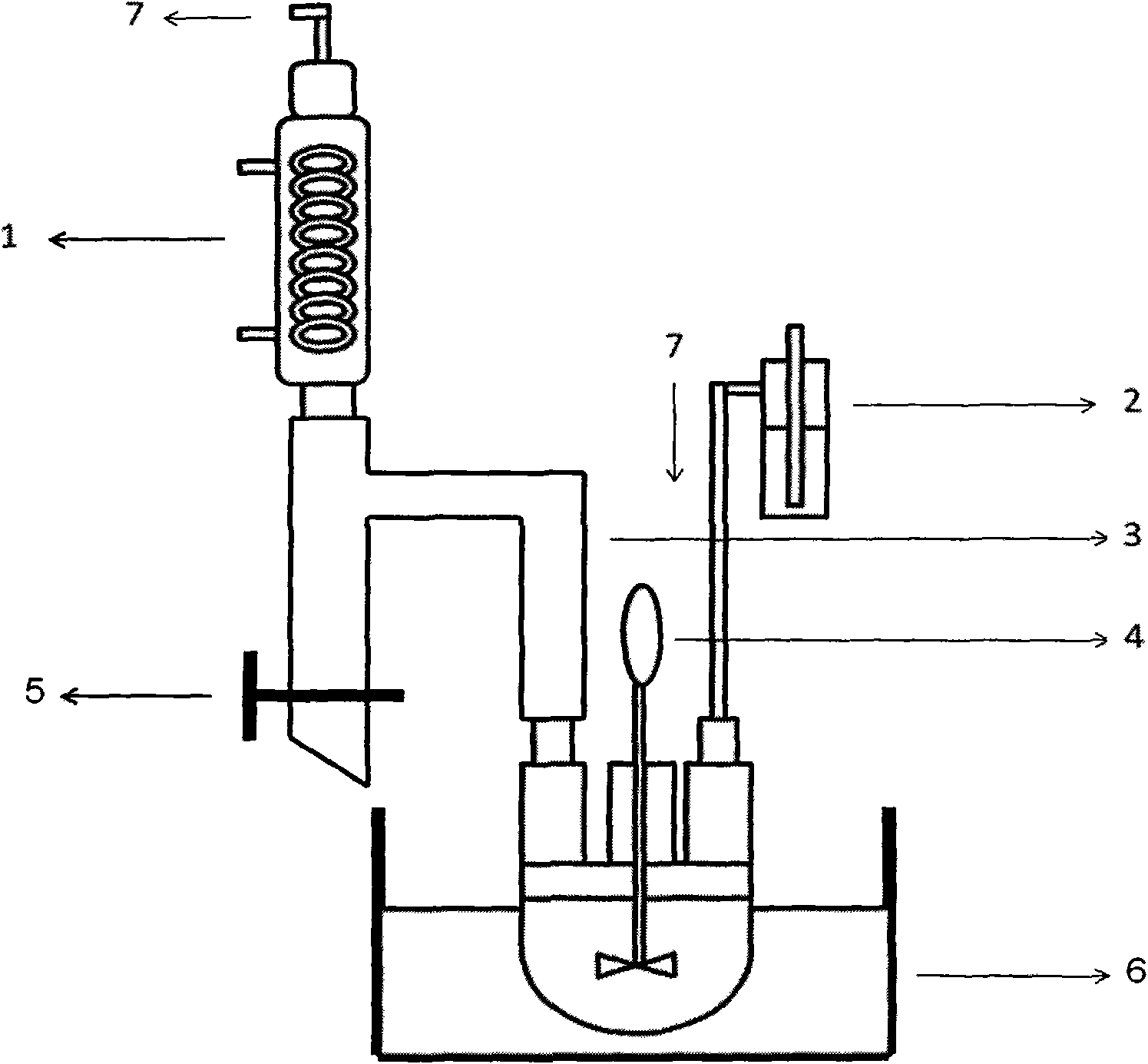
Image
Examples
Embodiment 1
[0062] Synthesis of lipophilic segment at the end of potassium phenate (taking the molecular weight of 5000g / mol as an example)
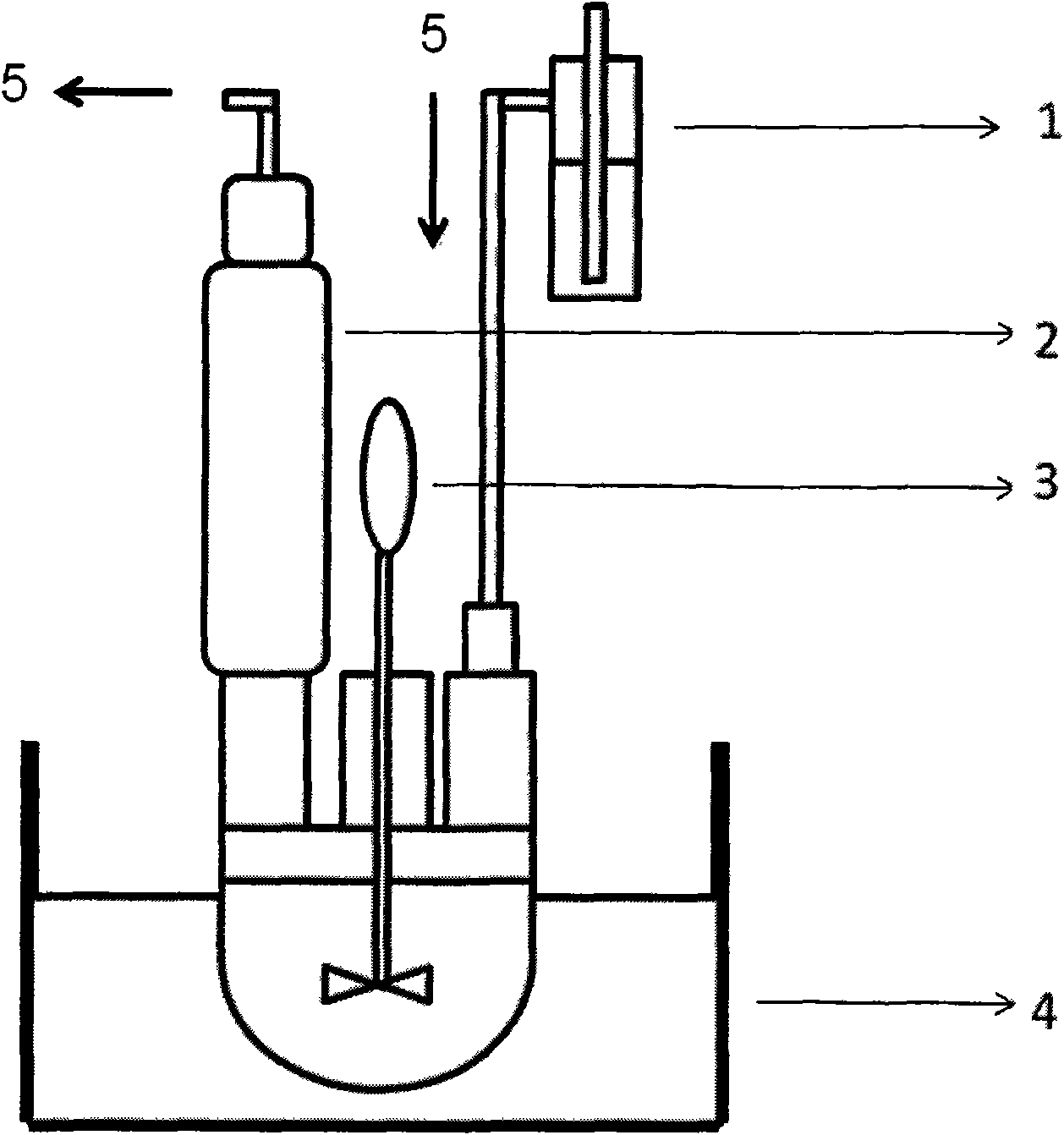
[0063] according to figure 2 In the shown reaction device, 4,4'-dichlorodiphenyl sulfone (DCDPS, 14.3580g, 50mmol), 4,4'-biphenol (BP, 10.0838g, 54.2mmol), anhydrous potassium carbonate ( K 2 CO 3 , 14.9820g, 108.4mmol), 122mL N,N-dimethylacetamide (DMAc) were mixed, heated to 180°C, and reacted for 24 hours. Stop heating and stirring, and cool to room temperature naturally. Suction filtration, the filtrate was poured into 800mL of methanol, a white powder was precipitated, filtered, washed with methanol, dried, and then vacuum-dried at 100°C for 24 hours to obtain a lipophilic fragment ending in potassium phenate, 20.4g, yield: 98%, Intrinsic viscosity: 0.22dL / g.
Embodiment 2
[0065] Synthesis of Chlorine-terminated Lipophilic Fragment (Taking a Molecular Weight of 5000g / mol as an Example)
[0066] according to figure 2 In the shown reaction device, 4,4'-dichlorodiphenylsulfone (DCDPS, 15.5641g, 54.2mmol), 4,4'-biphenyldiphenol (BP, 9.3105g, 50mmol), anhydrous potassium carbonate ( K 2 CO 3 , 13.8210g, 100mmol), 124mL N,N-dimethylacetamide (DMAc) were mixed, heated to 180°C, and reacted for 24 hours. Stop heating and stirring, and cool to room temperature naturally. Suction filtration, the filtrate was poured into 800mL methanol, a white powder was precipitated, filtered, washed with methanol, dried, and then vacuum-dried at 100°C for 24 hours to obtain a lipophilic fragment ending in potassium phenate, 20.6g, yield: 97%, Intrinsic viscosity: 0.24dL / g.
Embodiment 3
[0068] Synthesis of Hydrophilic Fragment with Potassium Phenate Tail (Taking Molecular Weight as 5000g / mol as an Example)
[0069] according to figure 2 In the shown reaction device, 3,3'-sodium disulfonate-4,4'-dichlorodiphenyl sulfone (SDCDPS, 24.5625g, 50mmol), 4,4'-biphenyldiphenol (BP, 11.0662g , 56.4mmol), anhydrous potassium carbonate (K 2 CO 3 , 15.5901g, 112.8mmol), 178mL of N-methylpyrrolidone (NMP) were mixed, heated to 180°C, and reacted for 48 hours. Stop heating and stirring, and cool to room temperature naturally. Suction filtration, the filtrate was poured into 1000mL isopropanol, white powder was precipitated, filtered, washed with isopropanol, dried, and then vacuum-dried at 100°C for 24 hours to obtain a hydrophilic fragment ending in potassium phenate, 30.1g, collected Rate: 94%, intrinsic viscosity: 0.18dL / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com