Preparation method of 5'-((5-methoxyl-2-phenyl indole-1-yl) methylene)-2'-oxo-3'-tetrahydrofurfuryl
A technology of methylenetetrahydrofuran and methoxymethoxyindole is applied in the directions of organic chemistry, drug combination, antitumor drugs, etc., and can solve the problems of difficult large-scale preparation, complicated steps, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
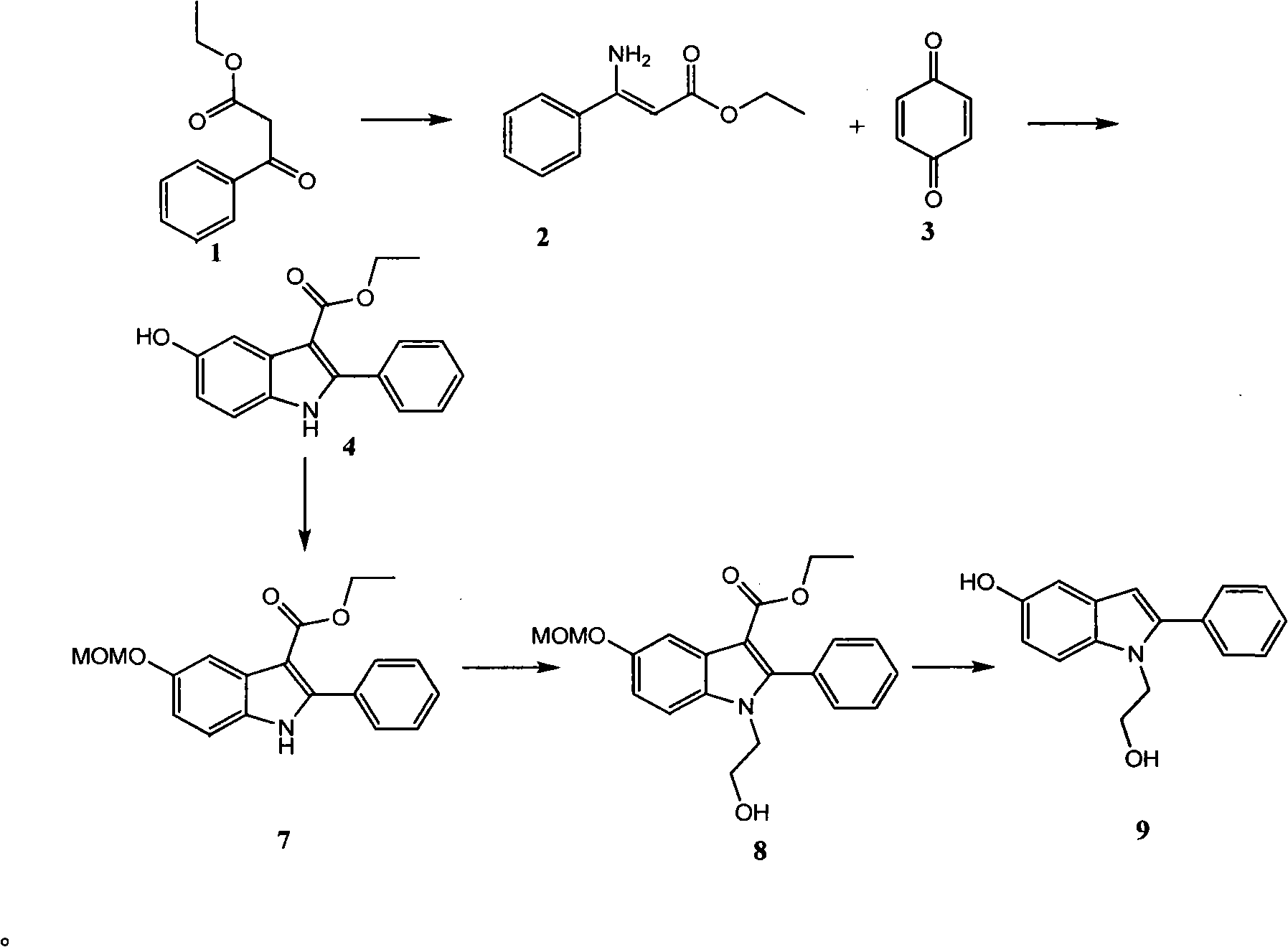
[0031] The preparation of embodiment 1 intermediate compound 9 (route B)
[0032] (1) Preparation of N-hydroxyethylamino-3-phenyl ethyl acrylate (compound 11)
[0033] 19.2g of ethyl benzoyl acetate 1 (0.1mol) and 6.71g of aminoethanol 10 (0.11mol) were mixed and dissolved in 80ml of benzene, 5 drops of concentrated hydrochloric acid were added dropwise, split flow dehydration for 13h, concentration, separation on a silica gel column (petroleum ether : ethyl acetate=3:1, volume ratio), to obtain 12.1 g of green oil, yield 51%. 1 H NMR (CDCl 3 ): δ8.63(s, 1H), 7.38(m, 5H), 4.67(s, 1H), 4.15(q, 2H, J=7.2), 3.62(t, 2H, J=6), 3.24(m , 2H), 1.28 (t, 3H, J=7.2).
[0034] (2) Preparation of ethyl 5-hydroxy-1-(2-hydroxyethyl)-2-phenyl-1H-indole-3-carboxylate (compound 12)
[0035] 2.664g of p-quinone and 2.7g of anhydrous zinc chloride were mixed in 74ml of dichloromethane, heated to boiling, then added 5.452g of the previous step product compound 11 in 25ml of dichloromethane sol...
Embodiment 2
[0038] The preparation of embodiment 2 intermediate compound 9 (route first):
[0039] 1, the preparation of 2-phenyl-5-oxindole (compound 5)
[0040] Compound 2 was obtained as described in the literature (J.Chem.Soc., Perkin Trans.1, 2002, 1663-1671), and then compound 2 and p-benzoquinone were prepared according to the operation of Example 1 to prepare compounds 12 and 9, to obtain Compound 5. The total yield of the above 3 steps is 38%. Melting point: 243-245°C. 1 H NMR (acetone-d 6 ): δ9.72 (s, 1H), 7.82-6.70 (m, 8H), 6.68 (s, 1H).
[0041] 2, Preparation of 2-phenyl-5-methoxymethoxyindole (compound 6)
[0042] 209 mg of compound 5 was dissolved in 15 ml of dry DMF, 45 mg of NaH was added, and reacted at room temperature for 5 min, then 0.1 ml of chloromethyl methyl ether was added, and stirring was continued at room temperature for 2 h. Add 30ml of cold water, extract with ether, dry the organic phase, and separate by column (petroleum ether:ethyl acetate=10:1, vol...
Embodiment 3
[0048] Example 3 5'-((5-methoxymethoxy-2-phenylindol-1-yl)methylene)-2'-oxo-3'-methylenetetrahydrofuran (S-10) preparation of
[0049] 1, Preparation of 2-(2-phenyl-5-methoxymethoxyindol-1-yl)ethanol (compound 13)
[0050] Dissolve 1.9 g of compound 9 in 30 ml of DMF, add 300 mg of NaH, stir at room temperature for 5 min, add 0.6 ml of chloromethyl methyl ether, and react at room temperature for 6 h. Terminate the reaction, add water and ethyl acetate, separate liquid extraction, wash the organic phase with water, dry, concentrate, silica gel column chromatography (petroleum ether: ethyl acetate = 4: 1, volume ratio) to give 950mg yellow oil 13, yield 80%. 1 H NMR (CDCl 3 ): δ7.54-6.97(m, 8H), 6.47(s, 1H), 5.21(s, 2H), 4.31-4.28(t, J=5.6Hz), 3.83-3.80(t, J=5.6Hz) , 3.53(s, 3H).
[0051] 2. Preparation of 2-(2-phenyl-5-methoxyindol-1-yl)acetaldehyde (14)
[0052] 215mg of compound 13 was dissolved in 20ml of DMSO, added 304mg of IBX, stirred at room temperature until clea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com