Bevantolol hydrochloride orally disintegrating tablet
A technology of bevanolol hydrochloride and orally disintegrating tablets, which is applied in the field of bevanolol hydrochloride orally disintegrating tablets and its preparation, can solve the problem of not finding bevanolol hydrochloride orally disintegrating tablets and not being able to perform better Therapeutic effect, inability to play a quick-acting role, etc., to achieve the effect of improving bioavailability, good taste, and convenient administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Recipe: 1000 tablets
[0048] Component Weight
[0049] Bevanolol Hydrochloride 50g
[0050] Citric acid 20g
[0051] Sodium bicarbonate 10g
[0052] Microcrystalline Cellulose 200g
[0053] Low-substituted hydroxypropyl cellulose 130g
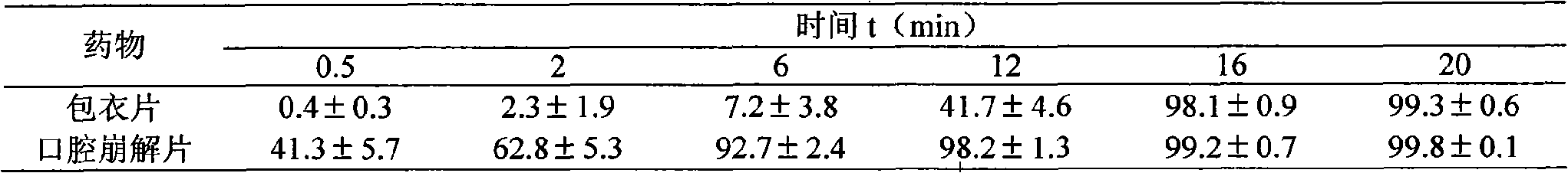
[0054] Preparation process: Weigh bevanolol hydrochloride, citric acid, microcrystalline cellulose and 50 g of low-substituted hydroxypropyl cellulose according to the prescription, put them into a mixer and mix them evenly, add 7% ethanol solution as a binder, and stir Evenly, granulate with a 40-mesh sieve, ventilate and dry at 50°C±5°C, pass the dry granules through a 40-mesh sieve for granulation, add the remaining amount of low-substituted hydroxypropyl cellulose to the granules, mix well, take a sample for testing, and pass the test. Tablets, ready to use. After the finished product passes the inspection, it is double-aluminum packaged and assembled.
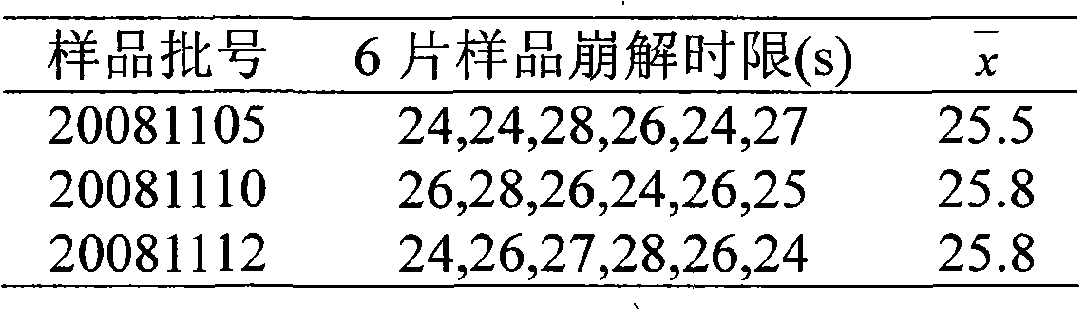
[0055] Test results of disintegration time limit of 6 samples in three b...
Embodiment 2
[0058] Recipe: 1000 tablets
[0059] Component Weight
[0060] Bevanolol Hydrochloride 100g
[0061] Citric acid 20g
[0062] Sodium bicarbonate 8g
[0063] Microcrystalline Cellulose 110g
[0064] Low-substituted hydroxypropyl cellulose 80g
[0065] Aspartame 40g
[0066] Micro Silica Gel 2g
[0067] Preparation process: Weigh bevanolol hydrochloride, citric acid, microcrystalline cellulose, and 40 g of low-substituted hydroxypropyl cellulose according to the prescription, put them into a mixer and mix them evenly, add 7% ethanol solution as a binder, Stir evenly, granulate with a 40-mesh sieve, ventilate and dry at 50°C±5°C, pass the dry granules through a 40-mesh sieve for granulation, add the remaining low-substituted hydroxypropyl cellulose and aspartame to the granules, and mix Evenly, add the micro silica gel into it, continue to mix, take a sample for testing, if it is qualified, press it into tablets, that is to say. After the finished product passes the inspe...
Embodiment 3
[0072] Recipe: 1000 tablets
[0073] Component Weight
[0074] Bevanolol Hydrochloride 200g
[0075] Citric acid 20g
[0076] Sodium bicarbonate 8g
[0077] Microcrystalline Cellulose 90g
[0078] Low-substituted hydroxypropyl cellulose 40g
[0079] Aspartame 20g
[0080] Micro Silica Gel 2g
[0081] Preparation process: Weigh bevanolol hydrochloride, citric acid, microcrystalline cellulose, and 20 g of low-substituted hydroxypropyl cellulose according to the prescription, put them into a mixer and mix them evenly, add 7% ethanol solution as a binder, Stir evenly, granulate with a 40-mesh sieve, ventilate and dry at 50°C±5°C, pass the dry granules through a 40-mesh sieve for granulation, add the remaining low-substituted hydroxypropyl cellulose and aspartame to the granules, and mix Evenly, add the micro silica gel into it, continue to mix, take a sample for testing, if it is qualified, press it into tablets, that is to say. After the finished product passes the inspec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com