Heterocyclyl-substituted anti-hypercholesterolemic compounds
A compound and multi-substitution technology, applied in animal repellents, plant growth regulators, botanical equipment and methods, etc., can solve problems such as reducing the incidence and mortality of coronary heart disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach A
[0053]
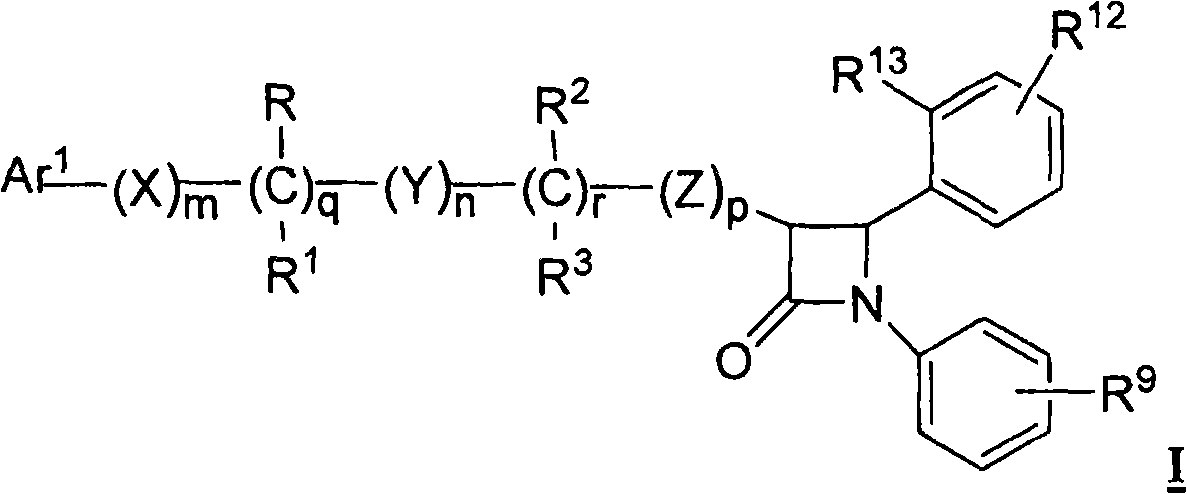
[0054] and pharmaceutically acceptable salts thereof, wherein variable (Ar 1 , R, R 1 , R 9 , R 12 , R 13 ) is as defined in Formula I or Embodiment A.
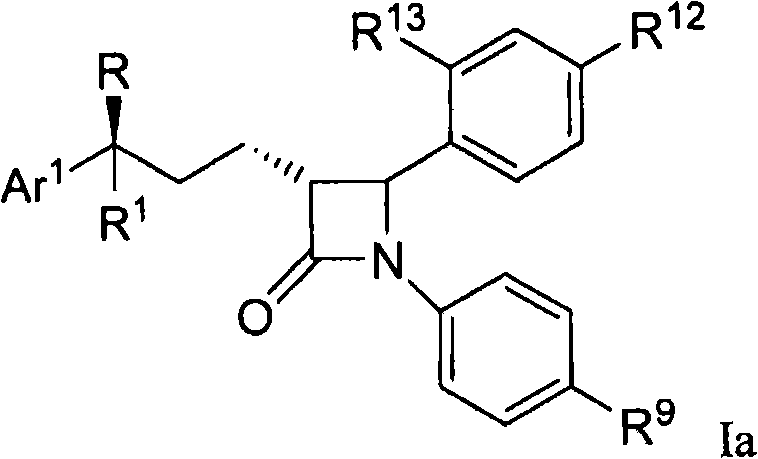
[0055] Another embodiment of the invention is a compound of formula I and embodiment A of formula Ib,
[0056]
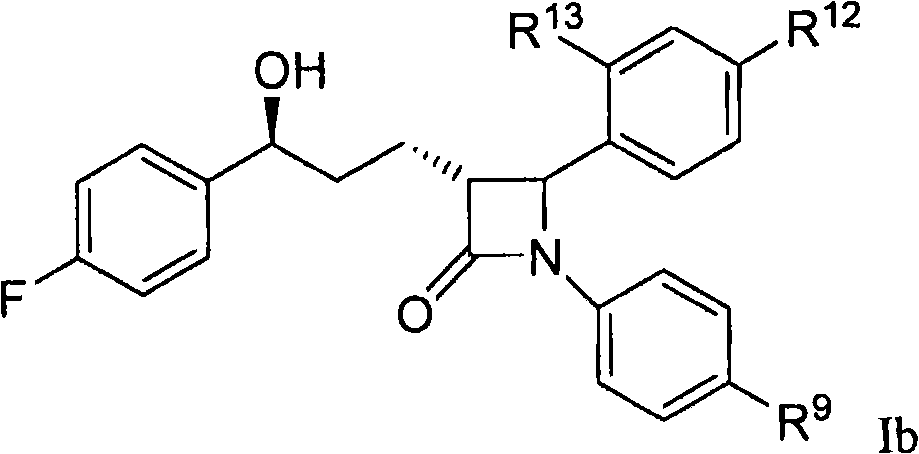
[0057] and pharmaceutically acceptable salts thereof, wherein the variable (R 9 , R 12 , R 13 ) is as defined in Formula I or Embodiment A.
[0058] Another embodiment of the invention is a compound of formula I, Ia or embodiment A, wherein Ar 1 selected from aryl and R 4 Substituted aryl, where R 4 are 1-2 substituents at each occurrence independently selected from the following: -OR 5 , -O(CO)R 5 , -O(CO)OR 8 ,-O-C 1-5 Alkyl-OR 5 , -O(CO)NR 5 R 6 , -NR 5 R 6 , -NR 5 (CO)R 6 , -NR 5 (CO)OR 8 , -NR 5 (CO)NR 6 R 7 , -NR 5 SO 2 R 8 、-COOR 5 ,-CONR 5 R 6 、-COR 5 、-SO 2 NR 5 R 6 ,-S(O) t R 8 ,-O-C 1-10 Alkyl-COOR 5 ,-O-C 1-10 Alkyl-CONR 5 R 6 and fluoro groups. ...
Embodiment 1
[0317] (3R,4S)-4-{4-[3,4-Dihydroxy-3-(hydroxymethyl)butyl]phenyl}-3-[(3S)-3-(4-fluorophenyl)- 3-Hydroxypropyl]-1-{4-[3-(1H-1,2,4-triazol-1-yl)propyl]phenyl}azetidin-2-one
[0318] Step A : Preparation of 4-{[(1E)-(4-iodophenyl)methylene]amino}phenol
[0319]
[0320] Under a nitrogen atmosphere, iodobenzaldehyde (400 g, 1.724 mol) was added to a round bottom flask, and the radical was dissolved in 2-propanol (950 ml). 4-Hydroxyaniline was added and the resulting mixture was heated to 70°C. After heating at this temperature for 3 h, a tan precipitate formed in the dark brown solvent mixture. The reaction mixture was cooled, filtered and washed with 2-propanol followed by diethyl ether. The organics were evaporated in vacuo and the residue was dried under high vacuum overnight to give the title compound which was used without further purification. 1 HNMR (500MHz, DMSO-D6) δ: 9.55(s, 1H), 8.59(s, 1H), 7.85(d, 2H), 7.63(d, 2H), 7.2(d, 2H), 6.80(d, 2H ).
[0321] St...
Embodiment 2
[0353] (3R,4S)-4-{4-[3,4-Dihydroxy-3-(hydroxymethyl)butyl]phenyl}-3-[(3S)-3-(4-fluorobenzene Base)-3-hydroxypropyl]-1-{4-[2-(1H-1,2,4-triazol-5-yl)ethyl]phenyl}azetidine-2- ketone
[0354] Step A : Acetic acid (1S)-3-((2S,3R)-2-(4-{4-(acetyloxy)-3-[(acetoxy)methyl]-3- Hydroxybut-1-yn-1-yl}phenyl)-4-oxo-1-{4-[(trimethylsilyl)ethynyl]phenyl} Preparation of azetidin-3-yl)-1-(4-fluorophenyl)propyl ester
[0355]
[0356] Bubble nitrogen through (1S)-3-[(2S,3R)-2-(4-{4-(acetyloxy)-3-[(acetoxy)methyl]-3-hydroxybutyrate -1-yn-1-yl}phenyl)-4-oxo-1-(4-{[(trifluoromethyl)sulfonyl]oxy}phenyl)azetidin-3-yl] -1-(4-fluorophenyl)propyl ester (9.77g, 12.8mmol; step F intermediate of Example 1), trimethylsilylacetylene (4.52mL, 32mmol), tetra-n-butylammonium iodide (4.72 g, 12.8 mmol) and triethylamine (8.92 mL, 64 mmol) in anhydrous DMF (100 mL) for 15 min. Add Pd(PPh 3 ) 4 (1.48g, 1.28mmol) and CuI (0.49g, 2.56mmol), the reaction mixture was heated at 50°C under nitrogen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com