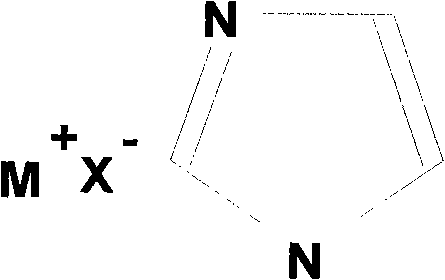
Binary eutectic ionic liquid and preparation method thereof
An ionic liquid and eutectic technology, applied in organic chemistry and other directions, can solve the problems of complex preparation process, high viscosity and high cost, and achieve the effects of simple preparation process, low material density and energy density, and good electrical conductivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Preparation of Tetrabutylammonium Bromide / Imidazolium Ionic Liquid
[0018] In the first step, imidazole and tetrabutylammonium bromide were purified by recrystallization from toluene, and dried under vacuum at 60°C to obtain pure imidazole and tetrabutylammonium bromide.
[0019] In the second step, weigh 9.67g (0.03mol) of tetrabutylammonium bromide and 4.77g (0.07mol) of imidazole respectively, mix well, and heat at 100°C under stirring to obtain a homogeneous colorless liquid, and then vacuum After drying, the binary deep eutectic ionic liquid tetrabutylammonium bromide / imidazole is obtained. According to the DSC test, the eutectic temperature of this ionic liquid is 21°C; the viscosity at 70°C is 27.8cp. The conductivity at 130°C is 1.9×10 -2 Scm -1 ; Stable electrochemical window is 3.4V.
Embodiment 2
[0021] Preparation of Choline Chloride / Imidazolium Ionic Liquid
[0022] In the first step, imidazole is purified by recrystallization from toluene, choline chloride is purified by recrystallization from acetone, and vacuum-dried at 60°C to obtain pure imidazole and choline chloride.
[0023] In the second step, choline chloride 4.19g (0.03mol) and imidazole 4.77g (0.07mol) were weighed respectively, mixed uniformly, and heated at 100°C under stirring to obtain a homogeneous colorless liquid, and then vacuum-dried at 60°C, That is, the binary deep eutectic ionic liquid choline chloride / imidazole is obtained. According to the DSC test, the eutectic temperature of this ionic liquid is 56° C.; the viscosity at 70° C. is 15.0 cp. Conductivity at 130°C 5.2×10 -2 Scm -1 ; Stable electrochemical window is 3.0V.
Embodiment 3
[0025] Preparation of 1-ethyl-3-butylbenzotriazole hexafluorophosphate / imidazole ionic liquid
[0026] The first step, the purification of raw materials and the synthesis of 1-ethyl-3-butylbenzotriazole hexafluorophosphate
[0027] ①Imidazole was purified by recrystallization from toluene, and vacuum-dried at 60°C to obtain pure imidazole.
[0028] ②Synthesis and purification of 1-ethyl-3-butylbenzotriazole hexafluorophosphate
[0029] 20g of benzotriazole and 20ml of bromobutane were dissolved in 30% NaOH aqueous solution, then 1g of tetrabutylammonium bromide was added, stirred at 50°C for 10h, left to stand, and the upper layer was rotary evaporated at 70°C to obtain 1-butyl Benzotriazole. Pour 30g of 1-butylbenzotriazole and 13ml of bromoethane into a 100ml three-neck flask at 70°C and stir and reflux for 48-72h, the product is washed three times with ethyl acetate, and rotary evaporated at 70°C for 3h to obtain 1-ethyl- 3-Butylbenzotriazole bromide. Disperse 25g of 1-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com