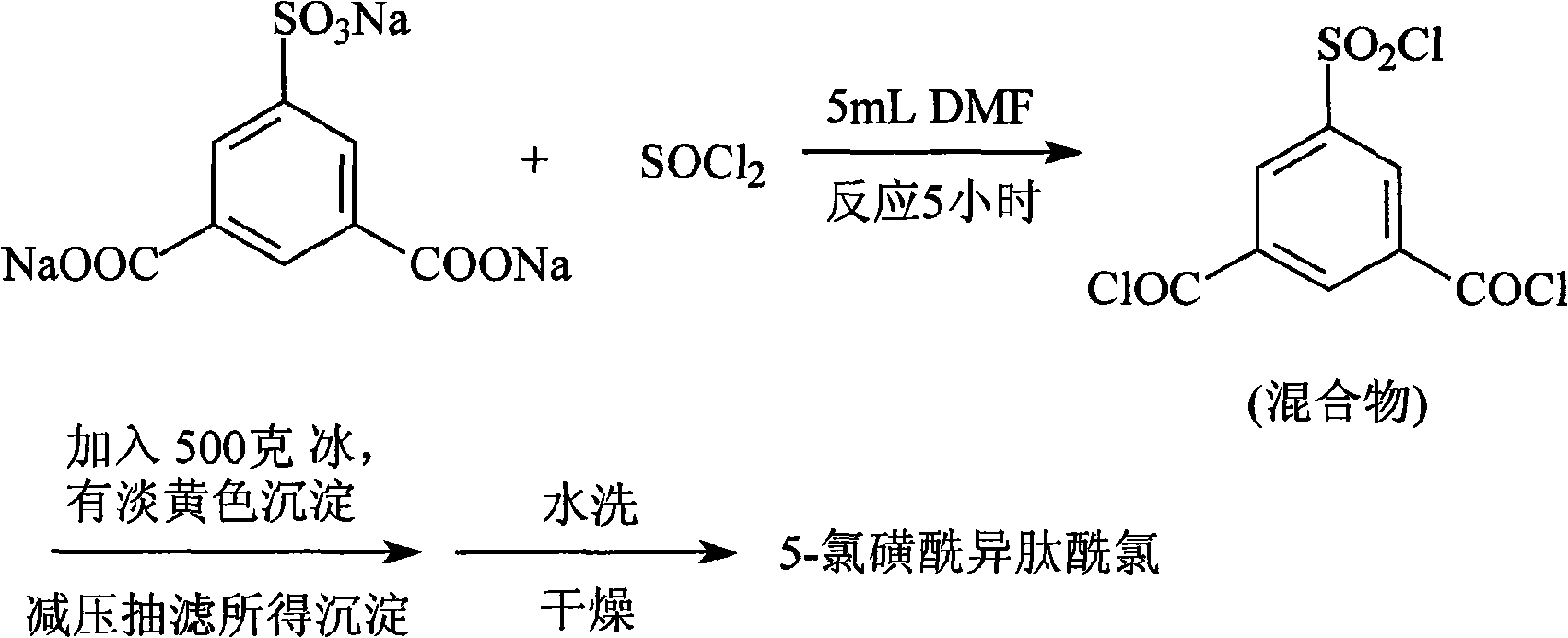
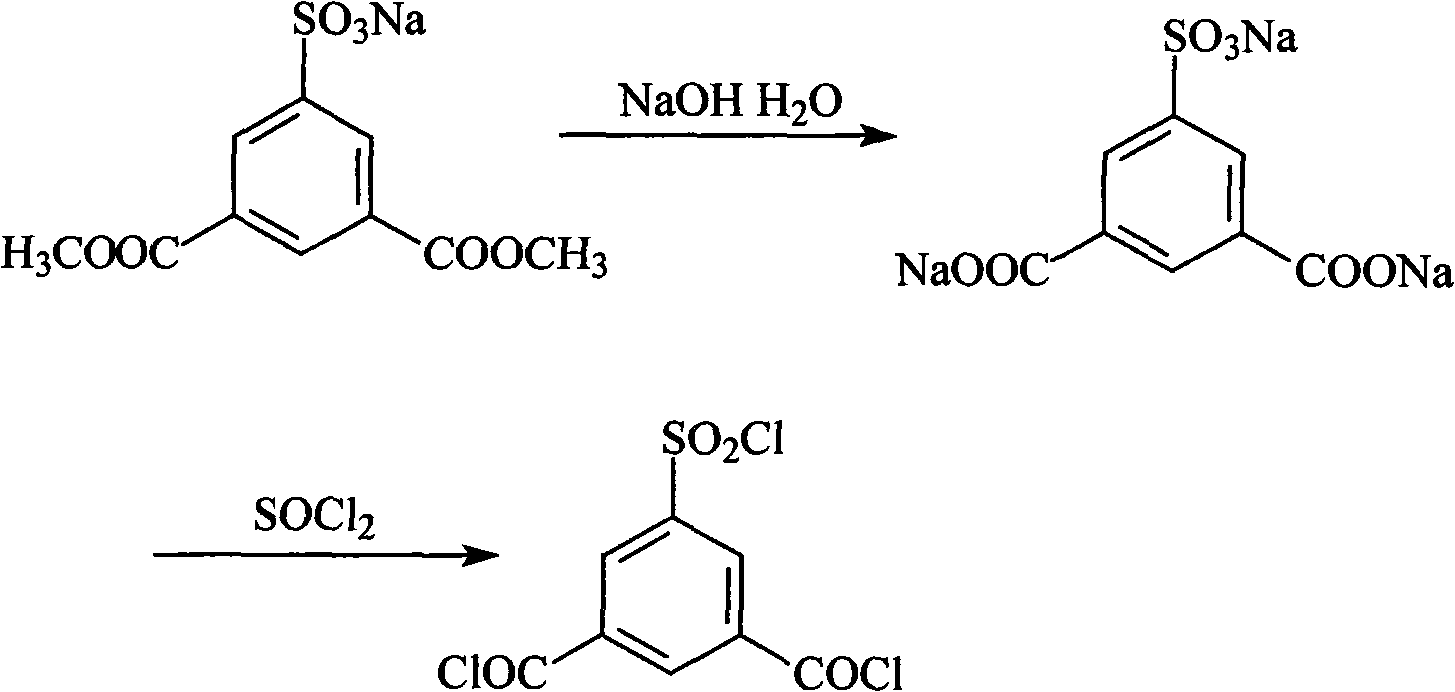
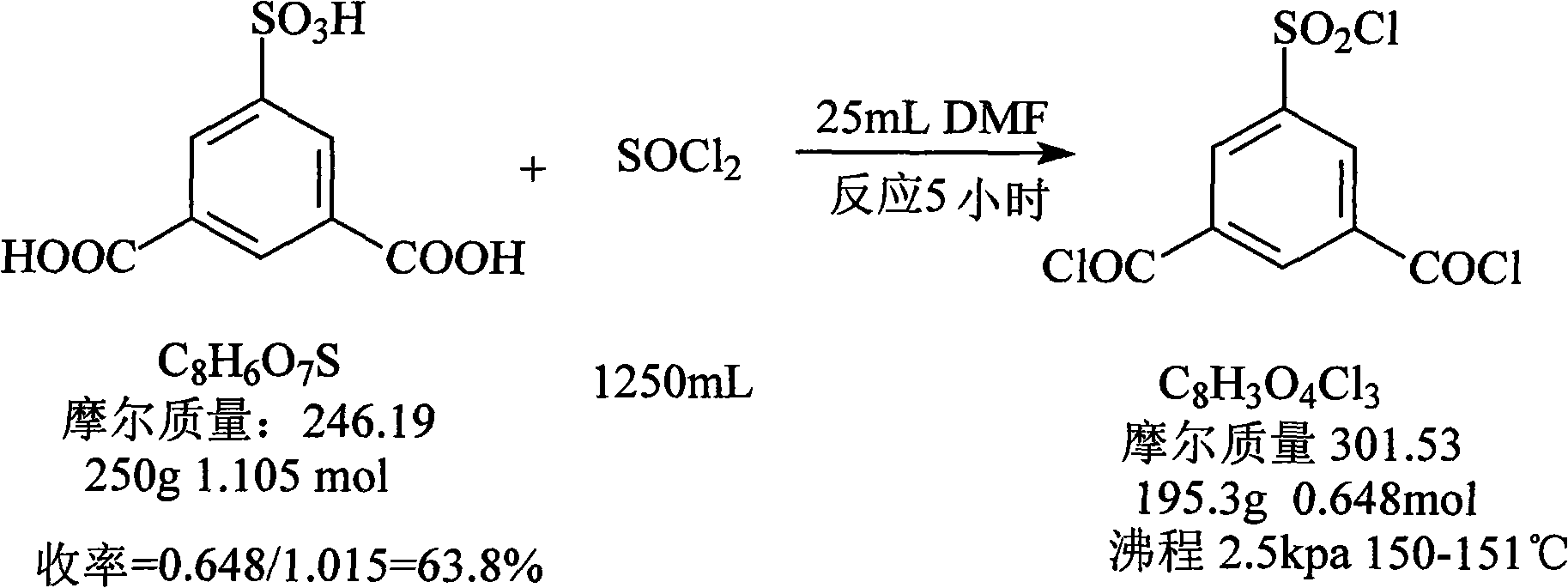
Preparation method of 5-chlorosulfonyl isophthaloyl acid chloride
A technology of chlorosulfonyl isophthaloyl chloride and chlorinating agent, which is applied in the preparation of sulfonic acid, organic chemistry, etc., can solve the research and application that affects the research and development of acyl chloride-based benzenesulfonyl chloride, the expensive acyl chloride agent, and the difficulty in post-processing and other problems, to achieve the effect of easy reaction, easy operation and easy conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In a 2-liter round-bottomed flask, a mixture of 3.6 moles of finely ground phosphorus pentachloride and 0.6 moles of sodium 3,5-dicarboxybenzenesulfonate previously dried at 140°C for three hours was placed. The mixture was heated on an oil bath at 180°C for 15 hours to reflux, and a reflux circulating water condenser was installed on the flask. During the heating period, the flask should be taken out of the oil bath every four hours, cooled for 15 minutes, stoppered, and shaken well until the reactant becomes a paste. At the end of the heating, cool the mixture, add 1 liter of water and 1 kg of crushed ice, and 5-chlorosulfonylisophthaloyl chloride will sink to the bottom of the bottle. After separation, wash with water once, extract with ether, and distill off most of the ether with a rotary evaporator. , placed in a vacuum oven for vacuum drying, the reaction mixture was transferred to a Clayson bottle, connected to a short air condenser, and gradually heated up for ...
Embodiment 2-4
[0035] The feeding amount of 3,5-dicarboxybenzene sodium sulfonate remains constant at 0.6 mole, changes the feeding amount of phosphorus pentachloride, other operating conditions are all the same as in Example 1, and the results are shown in the table below
[0036]
Embodiment 5
[0038] In a 2-liter round-bottomed flask, put a mixture of 3.6 moles of finely ground phosphorus pentachloride and 0.6 moles of 3,5-dicarboxybenzene sodium sulfonate that was previously dried at 140°C for three hours, and place the mixture in oil Heat on the bath at 180°C for 12 hours. Other reaction operating conditions are the same as in Example 4, and the resulting product yield is 51.6%, and the purity is 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com