Tenofovir disoproxil salt preparation method
A technology of tenofovir disoproxil and tenofovir, which is applied in the field of preparation of tenofovir disoproxil salt, can solve the problems that the content of a single impurity in the final product exceeds the standard, can only reach 36.5%, and the product cost is high. Improve market competitiveness, reduce residence time, improve the effect of purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
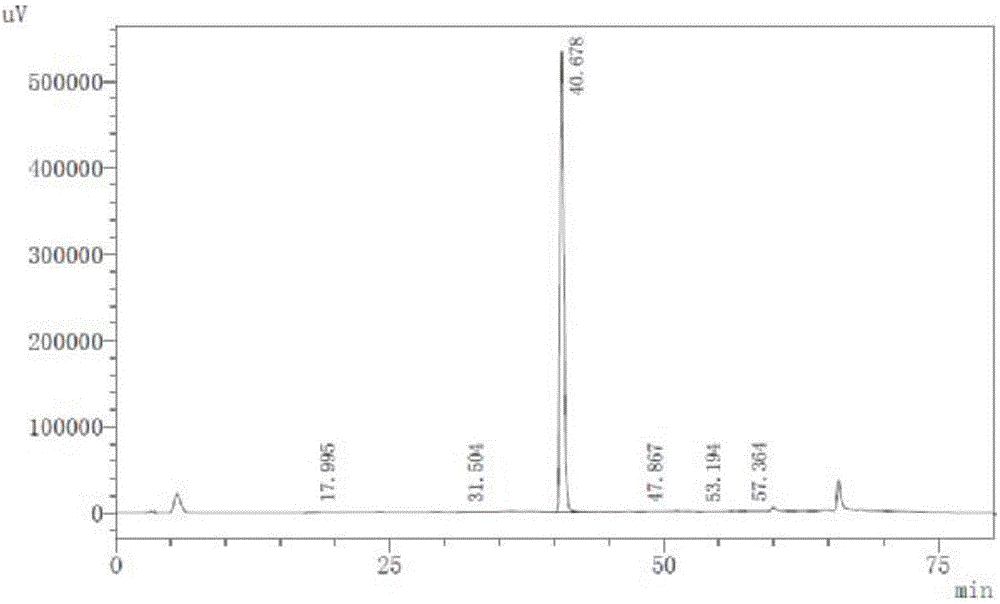
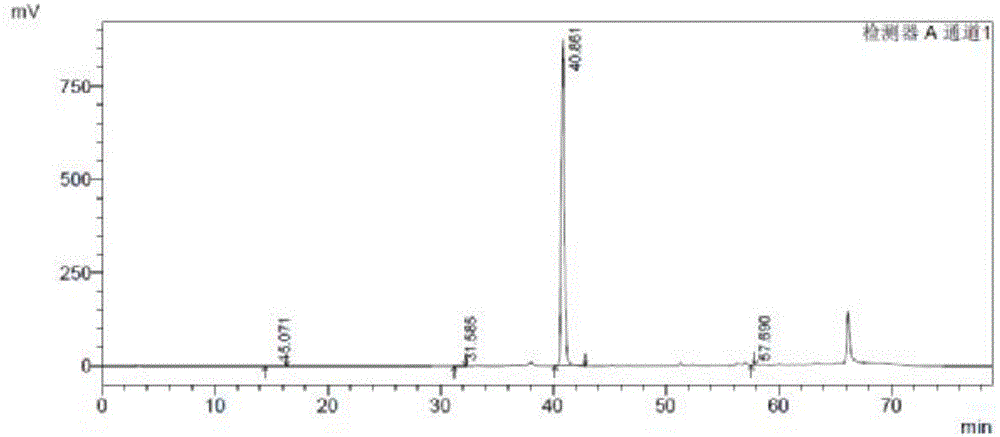
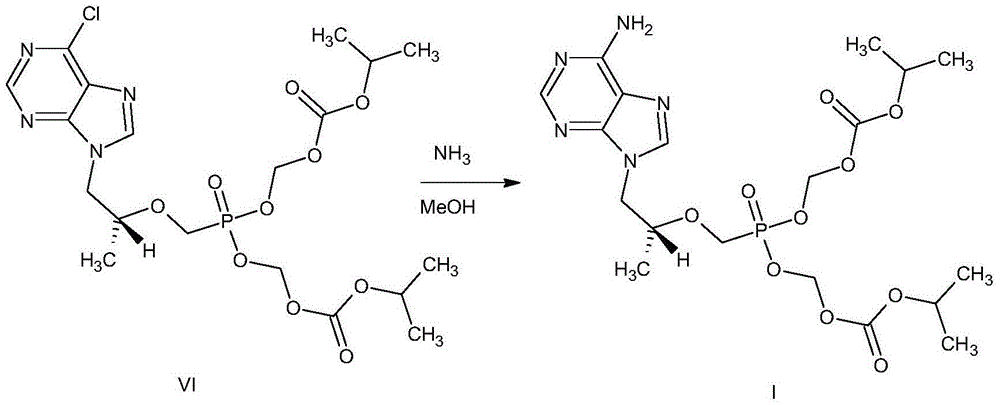
Image
Examples
Embodiment 1
[0029] (1) Control the temperature at 25°C to 35°C, N 2 Under protection, add 1000mL of N-methylpyrrolidone and 950g of chloromethyl isopropyl carbonate to a 5L four-neck flask successively, then slowly add 700mL of triethylamine dropwise, then add 40g of tetraethylammonium bromide, and heat up to 40°C ~45°C, add 400g of tenofovir or tenofovir monohydrate, continue to heat up to 50°C and react for 3h;
[0030] After the reaction is completed, cool down to 25°C to 30°C, filter the reactant with suction, rinse the filter cake with 4000mL of ethyl acetate, transfer the filtrate to a 20L four-necked flask, add 4800mL of tap water to the four-necked flask, The temperature was controlled at 25°C to 30°C, stirred for 5 minutes, and allowed to stand for 10 minutes to separate layers, the upper layer was ethyl acetate layer A1, and the lower layer was water layer B1;
[0031] The water layer B1 was extracted four times with ethyl acetate, using 1000 mL of ethyl acetate each time, stir...
Embodiment 2
[0040] (1) Control the temperature at 25°C to 35°C, N 2 Under protection, 1000mL of N,N-dimethylformamide and 950g of chloromethyl isopropyl carbonate were successively added into a 5L four-necked flask, then 700mL of triethylamine was slowly added dropwise, and then 38g of sodium lauryl sulfate was added. Raise the temperature to 40°C-45°C, add 400g of tenofovir or tenofovir monohydrate, and continue the reaction at 40°C-45°C for 5 hours;
[0041] After the reaction is completed, lower the temperature to 25°C to 30°C, filter the reactant with suction, rinse the filter cake with 3000mL isopropyl acetate, transfer the filtrate to a 20L four-necked flask, and add 4500mL of tap water to the four-necked flask , the temperature is controlled at 25°C to 30°C, stirred for 5 minutes, and allowed to stand for 10 minutes to separate layers, the upper layer is the isopropyl acetate layer A1, and the lower layer is the water layer B1;
[0042] Extract the water layer B1 with isopropyl ac...
Embodiment 3
[0051] (1) Control the temperature at 25°C to 35°C, N 2 Under protection, add 1000mL of dimethyl sulfoxide and 950g of chloromethyl isopropyl carbonate in sequence to a 5L four-neck flask, then slowly add 700mL of triethylamine dropwise, then add 40g of tetrabutylammonium bromide, and heat up to 40°C ~45°C, add 400g of tenofovir or tenofovir monohydrate, continue to heat up to 60°C, and then keep warm at this temperature for 2h;
[0052] After the reaction is completed, lower the temperature to 25°C to 30°C, filter the reactant with suction, rinse the filter cake with 4500mL of 1,2-chloroethane, transfer the filtrate to a 20L four-necked flask, and add 5000mL of tap water, the temperature is controlled at 25°C to 30°C, stirred for 5 minutes, and allowed to stand for 10 minutes to separate layers, the upper layer is 1,2-chloroethane layer A1, and the lower layer is water layer B1;
[0053] The aqueous layer B1 was extracted four times with 1,2-chloroethane, using 1200 mL of 1,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com