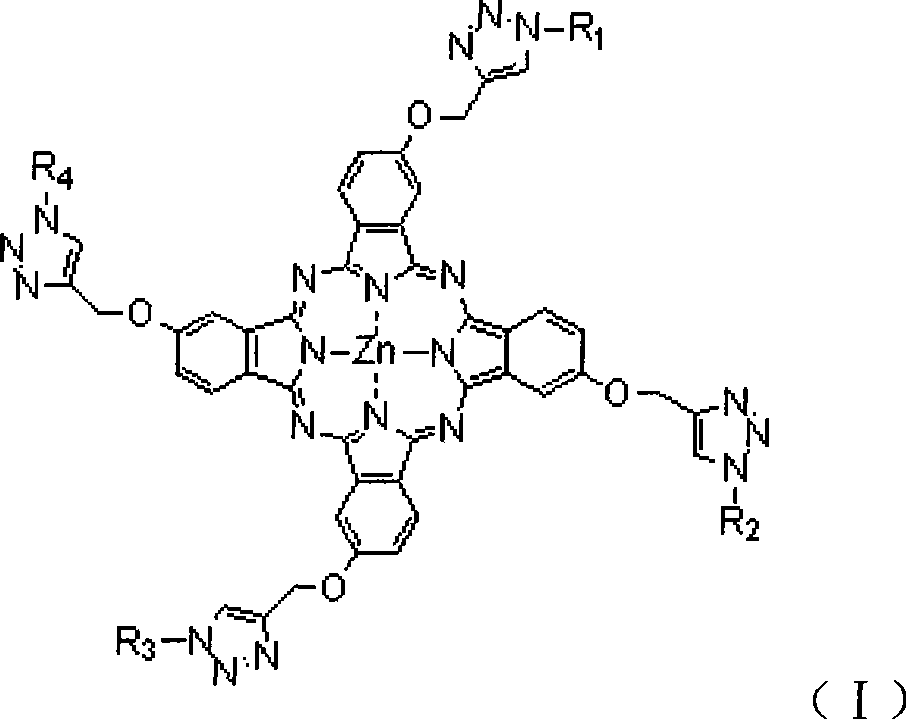
Water-soluble sugar phthalocyanine and a method for preparing same
A water-soluble sugar and sugar phthalocyanine technology, applied in the field of organic compound synthesis, can solve the problem of little improvement in the biocompatibility of phthalocyanine, and achieve good biocompatibility, good water solubility, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation method of water-soluble sugar phthalocyanine comprises the steps:
[0028] (1) Dissolve 1.638g of 4-chlorophthalonitrile, 1.2g of propynyl alcohol, and 8.3g of anhydrous potassium carbonate in 40ml of N,N-dimethylformamide (DMF), and react at 60°C for 1 sky;
[0029] Post-processing: Put ice cubes into a 1000ML large beaker, add about 500ML of distilled water, pour the reaction solution into the beaker, then add dichloromethane for extraction, combine the extracts, wash with water, combine the organic phases, concentrate by rotary evaporation, concentrate A small amount of anhydrous methanol was added to the solution for recrystallization to obtain alkynylphthalonitrile;
[0030] (2) 100 mg of alkynylphthalonitrile, 205 mg of azide-substituted glucose, 9 mg of anhydrous copper sulfate, and 22 mg of sodium ascorbate were dissolved in 15 methylene chloride and petroleum ether at a volume ratio of 4:1. In a mixed solvent, react with electromagnetic stirri...
Embodiment 2
[0036] The preparation method of water-soluble sugar phthalocyanine comprises the steps:
[0037] (1) Dissolve 1.6g of 4-nitrophthalonitrile, 1.2g of methyl butynol, and 8.3g of anhydrous potassium carbonate in 30ml of N,N-dimethylformamide, and react at 50°C for 3 day, pour it into a container filled with cold water, extract with chloroform, combine the extracts, wash with water, combine the organic phases, concentrate, add a small amount of anhydrous methanol to the concentrated solution for recrystallization, and obtain alkynylphthalonitrile;
[0038] (2) Dissolve 0.1g of alkynylphthalonitrile and 0.2g of azide-substituted fructose in 15ml of a mixed solvent of dichloromethane and petroleum ether at a volume ratio of 4:1, and stir at room temperature under the catalysis of 10mgCuBr React for 12 hours, wash the reaction solution with water, extract with chloroform, combine the organic phases, spin dry; obtain acetylated sugar phthalonitrile;
[0039] (3) Dissolve 0.6g of ac...
Embodiment 3
[0042] The preparation method of water-soluble sugar phthalocyanine comprises the steps:
[0043] (1) Dissolve 3.2g of 4-iodophthalonitrile, 2.4g of propargylamine, and 32.6g of anhydrous sodium carbonate in 50ml of N,N-dimethylformamide, react at 80°C for 1 day, pour In a container filled with cold water, extract with dichloromethane, combine the extracts, wash with water, combine the organic phases, concentrate, add a small amount of anhydrous methanol to the concentrated solution for recrystallization, and obtain alkynylphthalonitrile;
[0044](2) Dissolve 0.2g of alkynylphthalonitrile and 0.4g of azide-substituted galactose in 30ml of a mixed solvent of dichloromethane and petroleum ether at a volume ratio of 4:3, under the catalysis of 20mgCuCl, at room temperature Stir the reaction for 72 hours, wash the reaction solution with water, extract with dichloromethane, combine the organic phases, and spin dry; obtain acetylated sugar phthalonitrile;
[0045] (3) Dissolve 0.8g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com