Method for preparing protamine and dextran sulfate sodium microcapsule
A technology of dextran sodium sulfate and protamine, which is applied in the direction of microcapsule preparation and microsphere preparation, can solve the problems of polyelectrolyte non-degradability or template toxicity residue, harsh preparation conditions, application restrictions, etc., and is easy to promote and application, easy to obtain, cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
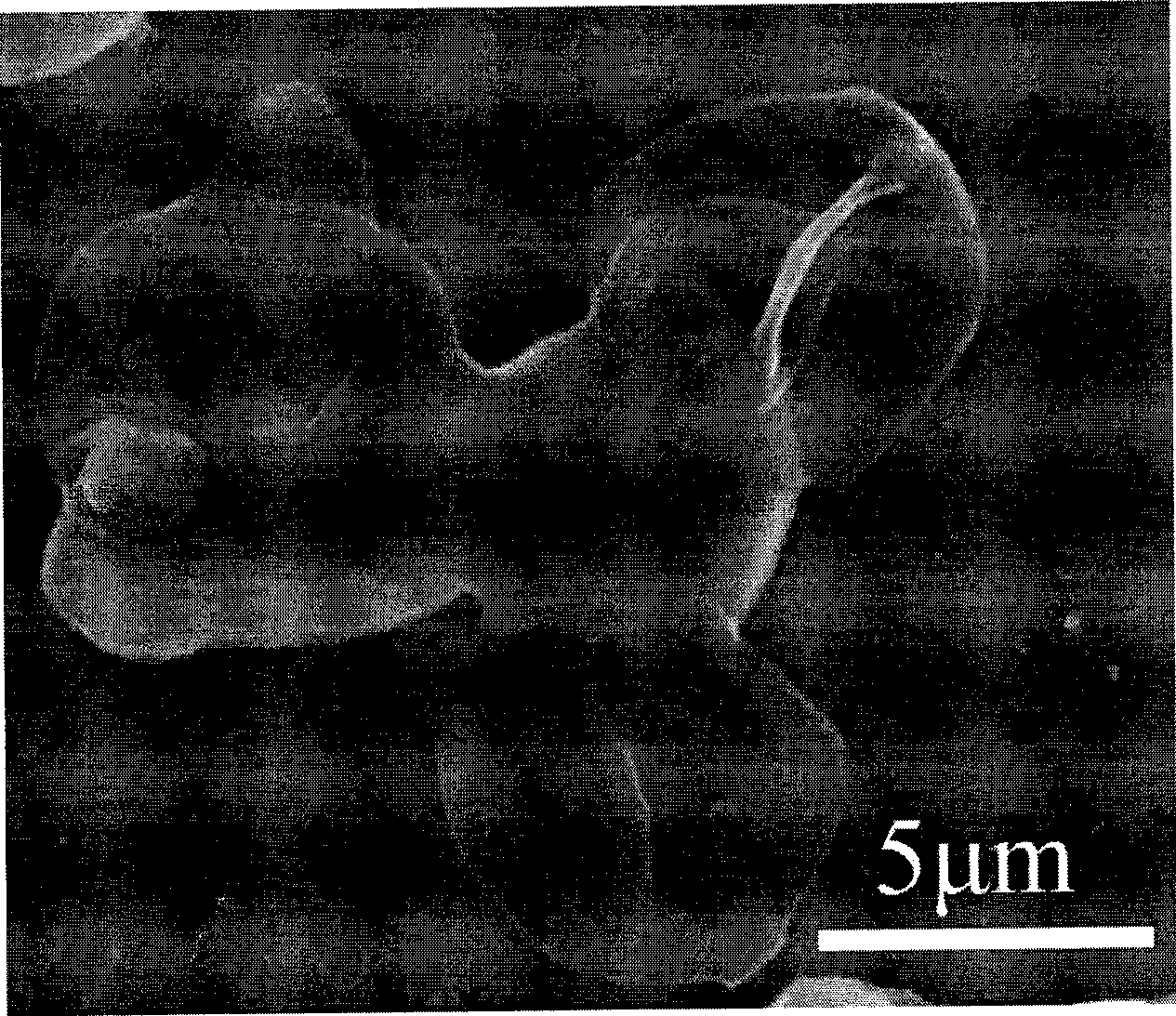
[0022] (1), first mix the calcium chloride solution of 33.3 g / liter and the sodium polystyrene sulfonate solution of 1 g / liter, wherein the mass ratio of calcium chloride and sodium polystyrene sulfonate is 17:1, and then Under stirring at a rotating speed of 600rpm, the sodium carbonate solution having the same molarity and volume as the calcium chloride was quickly added to the calcium chloride solution, and reacted at a constant temperature of 10° C. for 6 minutes to obtain calcium carbonate microspheres. The calcium carbonate microspheres were centrifuged and washed for later use. The obtained microspheres are regular spherical, such as figure 1 Shown is the morphology of calcium carbonate microspheres, as can be seen from the figure, the calcium carbonate microspheres have good monodispersity, and the particle size is about 4 microns.
[0023] (2) Add calcium carbonate microspheres to 2 g / L protamine sulfate aqueous solution containing 23.4 g / L sodium chloride, disperse ...
Embodiment 2
[0028] (1), first mix the calcium chloride solution of 33.3 g / liter and the sodium polystyrene sulfonate solution of 1 g / liter, wherein the mass ratio of calcium chloride and sodium polystyrene sulfonate is 50:1, and then Under stirring at a rotating speed of 600rpm, the sodium carbonate solution having the same molarity and volume as the calcium chloride was quickly added to the calcium chloride solution, and reacted at a constant temperature of 50° C. for 6 minutes to obtain calcium carbonate microspheres. The calcium carbonate microspheres were centrifuged and washed for later use. The obtained microspheres are regular spherical, and the particle size is about 4.5 microns.
[0029] (2) Add calcium carbonate microspheres to 2 g / L protamine sulfate aqueous solution containing 23.4 g / L sodium chloride, disperse evenly, then place on a shaker to vibrate for 20-30 minutes, centrifuge, and drain The supernatant was dispersed with deionized water, then centrifuged, and washed wit...
Embodiment 3
[0034] (1), first mix the calcium chloride solution of 33.3 g / liter and the sodium polystyrene sulfonate solution of 1 g / liter, wherein the mass ratio of calcium chloride and sodium polystyrene sulfonate is 100:1, and then Under stirring at a rotating speed of 600rpm, the sodium carbonate solution having the same molarity and volume as the calcium chloride was quickly added to the calcium chloride solution, and reacted at a constant temperature of 80° C. for 6 minutes to obtain calcium carbonate microspheres. The calcium carbonate microspheres were centrifuged and washed for later use. The obtained microspheres are regular spherical, and the particle size is about 5 microns.
[0035] (2) Add calcium carbonate microspheres to 2 g / L protamine sulfate aqueous solution containing 23.4 g / L sodium chloride, disperse evenly, then place on a shaker to vibrate for 20-30 minutes, centrifuge, and drain The supernatant was dispersed with deionized water, then centrifuged, and washed with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com