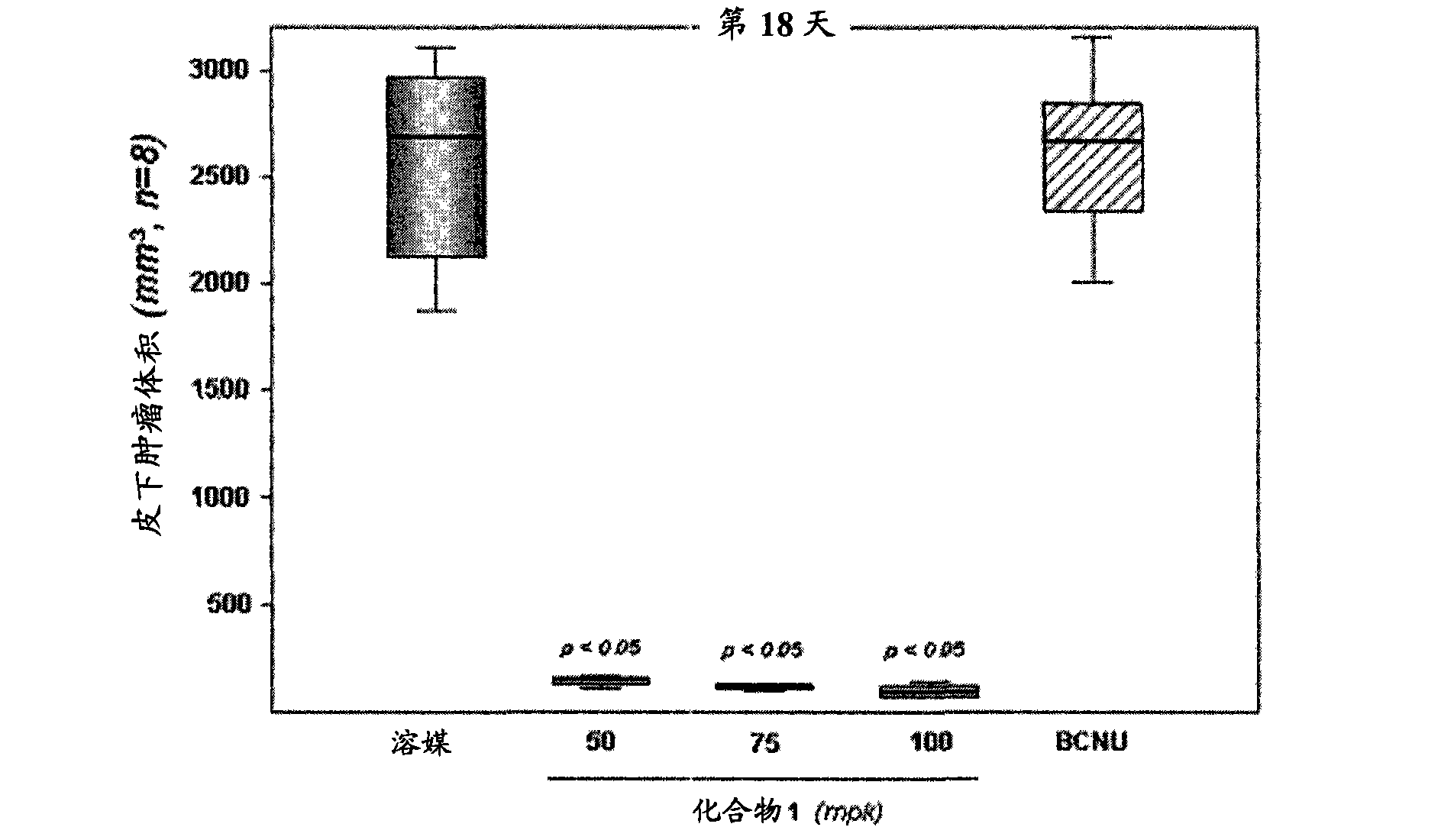
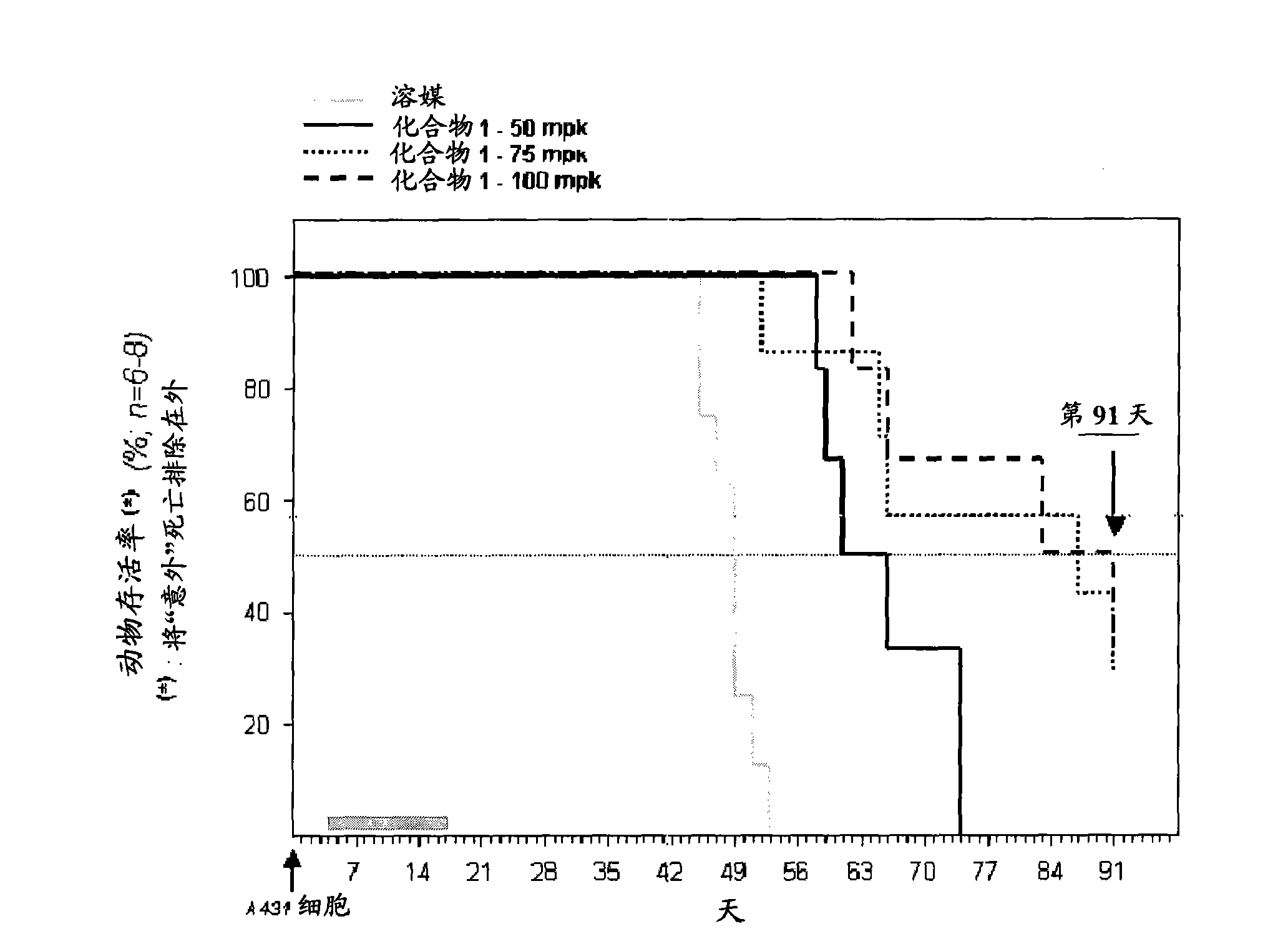
Use of a mt kinase inhibitor for treating or preventing brain cancer
A brain cancer, use technology, applied in the field of use of MT kinase inhibitors for the treatment or prevention of brain cancer, can solve the problems of normal tissue toxicity, unaware side effects, mechanical damage or destruction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] a) Preparation of 1-pentanol, 5-[[(4-bromo-2-nitrophenyl)methyl]amino]-(Intermediate 1)
[0040] Under RT, stir 4-bromo-2-nitro-benzaldehyde (0.013mol), 5-amino-1-pentanol (0.013mol) and tetrakis (2-propanolate) titanium (titanium, tetrakis (2- propanolato)) (0.014mol) in EtOH (15ml) for 1 hour, then heated the reaction mixture to 50°C and stirred for another 30min. Cool the mixture to RT and add NaBH in portions 4 (0.013 mol). The reaction mixture was stirred overnight, then poured into ice water (50ml). The resulting mixture was stirred for 20 min, the formed precipitate was filtered off (obtaining filtrate (I)), and washed with H 2 O washed, stirred in DCM (to dissolve product and remove it from Ti salt). The mixture was filtered, and the filtrate was dried (MgSO 4 ) and filtered, and finally the solvent was evaporated. Filtrate (I) was evaporated until EtOH was removed and the aqueous concentrate was extracted twice with DCM. The organic layer was separated a...
Embodiment 2
[0042] a) Preparation of 1-pentanol, 5-[[(4-bromo-2-nitrophenyl)methyl]methylamino]-(Intermediate 2)
[0043] A solution of intermediate 50 (0.0047 mol), formaldehyde (0.025 mol) and tetrakis(2-propyl titanate) (0.0051 mol) in EtOH (150 ml) was heated to 50 °C and stirred for 1 hour, then added in portions at RT NaBH 4 (0.026 mol). The reaction mixture was stirred overnight and quenched with water (100ml). The resulting mixture was stirred for 1 hour; the precipitate formed was filtered off and washed. The organic filtrate was concentrated and the aqueous concentrate was extracted with DCM and dried. The solvent was evaporated and the residue was filtered through silica gel (eluent: DCM / CH 3 OH from 98 / 2 to 95 / 5). The product fractions were collected and the solvent was evaporated, yielding 0.5 g of intermediate 2.
[0044] b) Preparation of 1-pentanol, 5-[[(4-bromo-2-nitrophenyl)methyl]methylamino]-, acetate (ester) (intermediate 3)
[0045] A solution of intermediate ...
Embodiment 3
[0053] a) 4,6-linked methylene (ethanediylidene) pyrimido [4,5-b] [6,1,12] benzoxadiazepine-pentadecene, 17-bromo-8,9 , 10, 11, 12, 13, 14, 19-Octahydro-20-methoxy-13-methyl-(Compound MTKI1) Preparation
[0054] At RT, a solution of Intermediate 6 (0.0011mol) in THF (50ml) was stirred, and tributylphosphine (0.0016mol) was added, followed by 1,1'-(azobiscarbonyl)bis-piperidine (0.0016mol) , and the reaction mixture was stirred for 2 hours. The solvent was evaporated to 1 / 3 of the original volume. The resulting precipitate was filtered off and washed. The filtrate was evaporated and the residue was purified by RP HPLC. The product fractions were collected and the organic solvent was evaporated. The aqueous concentrate was extracted twice with DCM and dried (MgSO4 ) organic layer, and then filtered. At 50°C, the solvent was evaporated and the residue was dried (vacuum), yielding 0.004 g (0.8%) of compound MTKI1.
[0055] To prepare the aforementioned pharmaceutical composi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com