Liquid-phase catalytic oxidation cycle method for preparing humic acid from coal residue
A technology of liquid-phase catalysis and oxidation cycle, applied in the field of applied chemistry, can solve problems such as excessive oxidation reaction, achieve the effects of reducing emissions, improving humic acid yield and resource utilization, and protecting the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
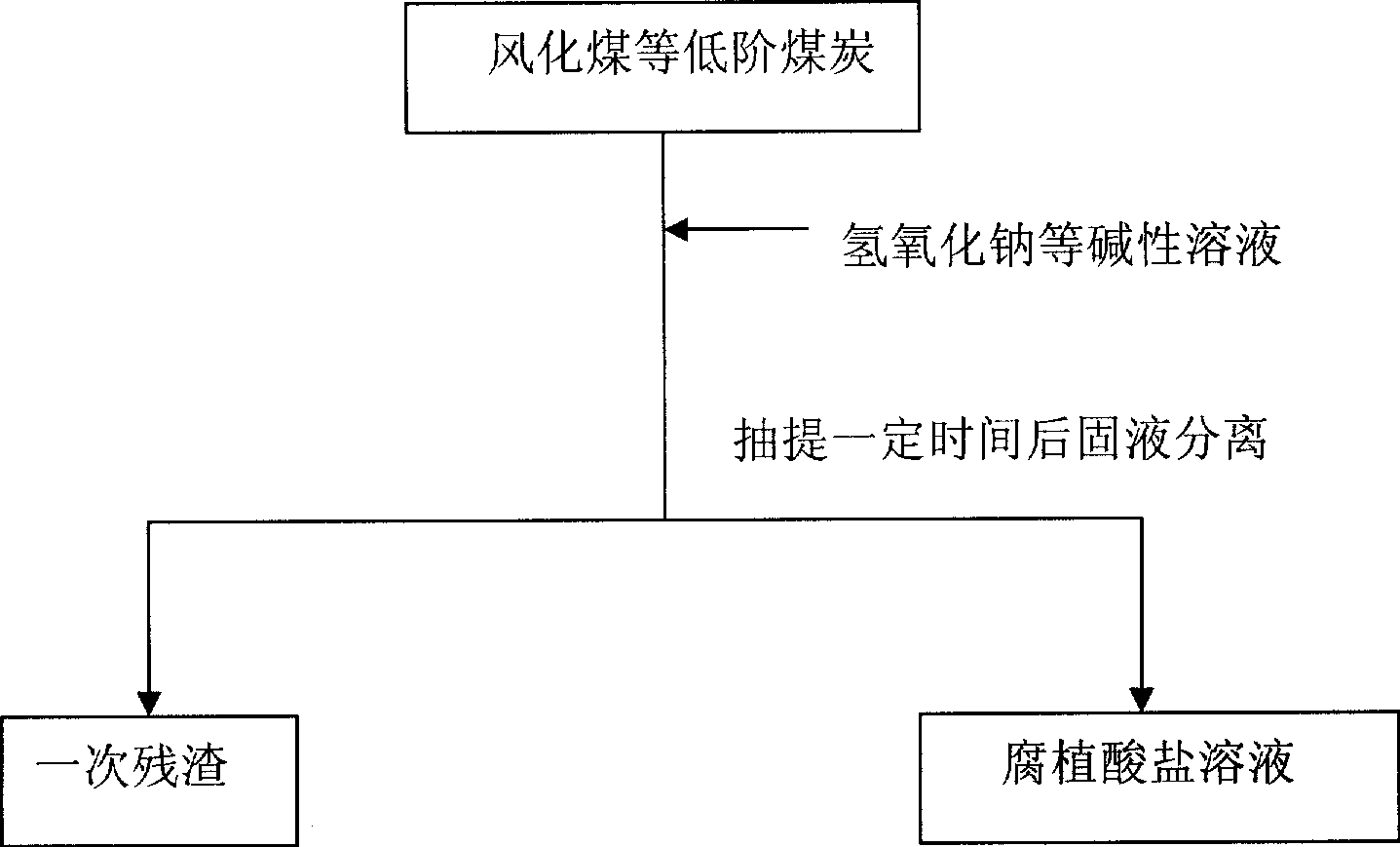
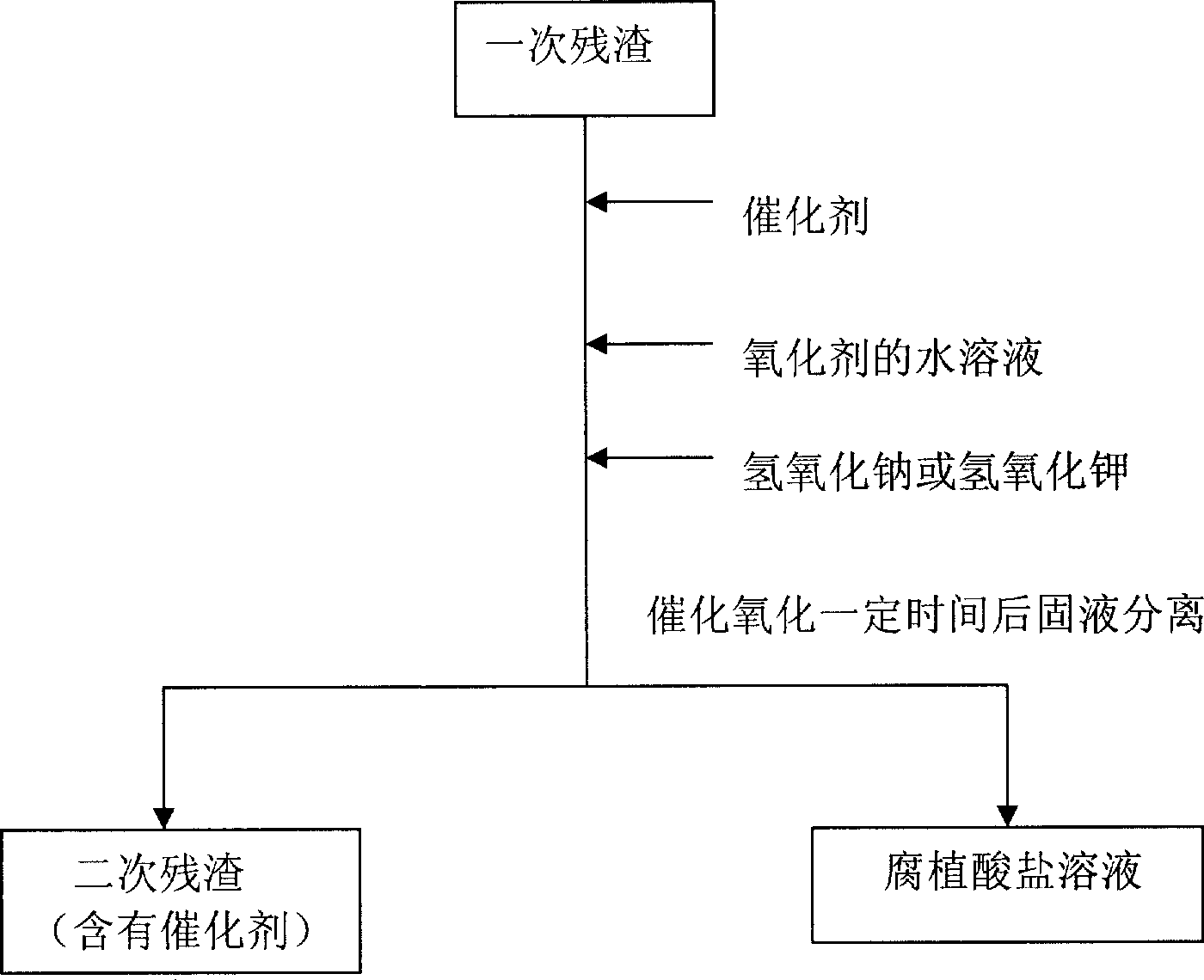
[0014] (1) At room temperature, add 1 kilogram of weathered coal humic acid extraction residue (called primary residue) to the reaction vessel, then add 1.7 grams of ferric oxide catalyst and 33 grams of solid sodium hydroxide, then add 3.3 liters of 0.6 % hydrogen peroxide, stirred and reacted for 1 hour and then separated from solid and liquid to obtain humic acid solution and secondary residue respectively. (2) 33 grams of solid sodium hydroxide and 3.3 liters of 0.6% hydrogen peroxide were added to the secondary residue, and the solid-liquid separation was carried out after stirring for 1 hour to obtain the humic acid solution and the tertiary residue respectively. (3) Add 33 grams of solid sodium hydroxide and 3.3 liters of 0.6% hydrogen peroxide to the three residues, stir the reaction for 1 hour and separate the solid and liquid to obtain humic acid solution and four residues respectively. (4) Add 33 grams of solid sodium hydroxide and 3.3 liters of 0.6% hydrogen peroxi...
Embodiment 2
[0016] (1) At room temperature, add 1 kilogram of weathered coal humic acid extraction residue (called primary residue) to the reaction vessel, then add 1.7 grams of copper oxide catalyst and 33 grams of solid sodium hydroxide, then add 3.3 liters of 0.6% Hydrogen peroxide, stirred and reacted for 1 hour, solid-liquid separation, and humic acid solution and secondary residue were obtained respectively. (2) 33 grams of solid sodium hydroxide and 3.3 liters of 0.6% hydrogen peroxide were added to the secondary residue, and the solid-liquid separation was carried out after stirring for 1 hour to obtain the humic acid solution and the tertiary residue respectively. (3) Add 33 grams of solid sodium hydroxide and 3.3 liters of 0.6% hydrogen peroxide to the three residues, stir the reaction for 1 hour and separate the solid and liquid to obtain humic acid solution and four residues respectively. (4) Add 33 grams of solid sodium hydroxide and 3.3 liters of 0.6% hydrogen peroxide to th...
Embodiment 3
[0018] (1) At room temperature, add 1 kilogram of weathered coal humic acid extraction residue (called primary residue) to the reaction vessel, then add 6.7 grams of zinc oxide catalyst and 33 grams of solid sodium hydroxide, then add 3.3 liters of 0.6% Hydrogen peroxide, stirred and reacted for 1 hour, solid-liquid separation, and humic acid solution and secondary residue were obtained respectively. (2) 33 grams of solid sodium hydroxide and 3.3 liters of 0.6% hydrogen peroxide were added to the secondary residue, and the solid-liquid separation was carried out after stirring for 1 hour to obtain the humic acid solution and the tertiary residue respectively. (3) Add 33 grams of solid sodium hydroxide and 3.3 liters of 0.6% hydrogen peroxide to the three residues, stir the reaction for 1 hour and separate the solid and liquid to obtain humic acid solution and four residues respectively. The above three consecutive liquid-phase catalytic oxidation residues make 68% of the organ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com