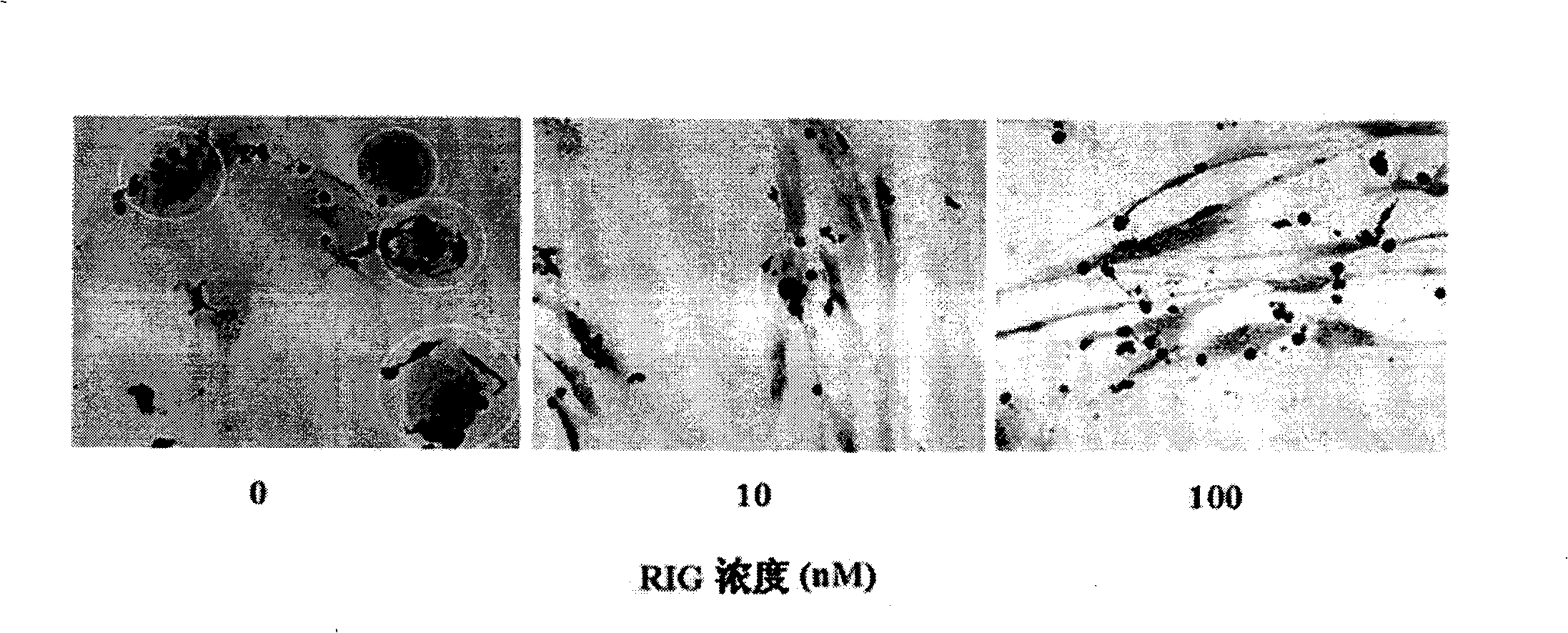
RANKL-Fc fusion protein, preparation method and application thereof
A technology of fusion protein and protein, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 Expression of fusion protein RANKL-Fc
[0043] The expression process of the RANKL-Fc fusion protein comprises the following steps: synthesizing the gene; inserting the gene into an expression vector, transfecting CHO cells with the expression vector; utilizing Hygromycin to screen and express the CHO engineering cell line of the RANKL-Fc fusion protein, cultivating the cell line, and finally from The RANKL-Fc protein was isolated and purified from the cell culture medium. Specifically include:
[0044] 1) Gene synthesis
[0045] Entrust a professional company of gene synthesis (Shanghai Sangon Bioengineering Technology Service Co., Ltd.) to synthesize the nucleotide sequence shown in SEQ ID NO: 5, in which 5-10 is the restriction site of Nhe I; 11-73 is the encoding Igκ The nucleotide sequence of chain secretion signal peptide; 74-823 is the nucleotide sequence of coding human RANKL extracellular region, and the RANKL part of its coding corresponds to 68...
Embodiment 2
[0052] Example 2 RANKL-Fc fusion protein and RANK (RANKL receptor) binding experiment
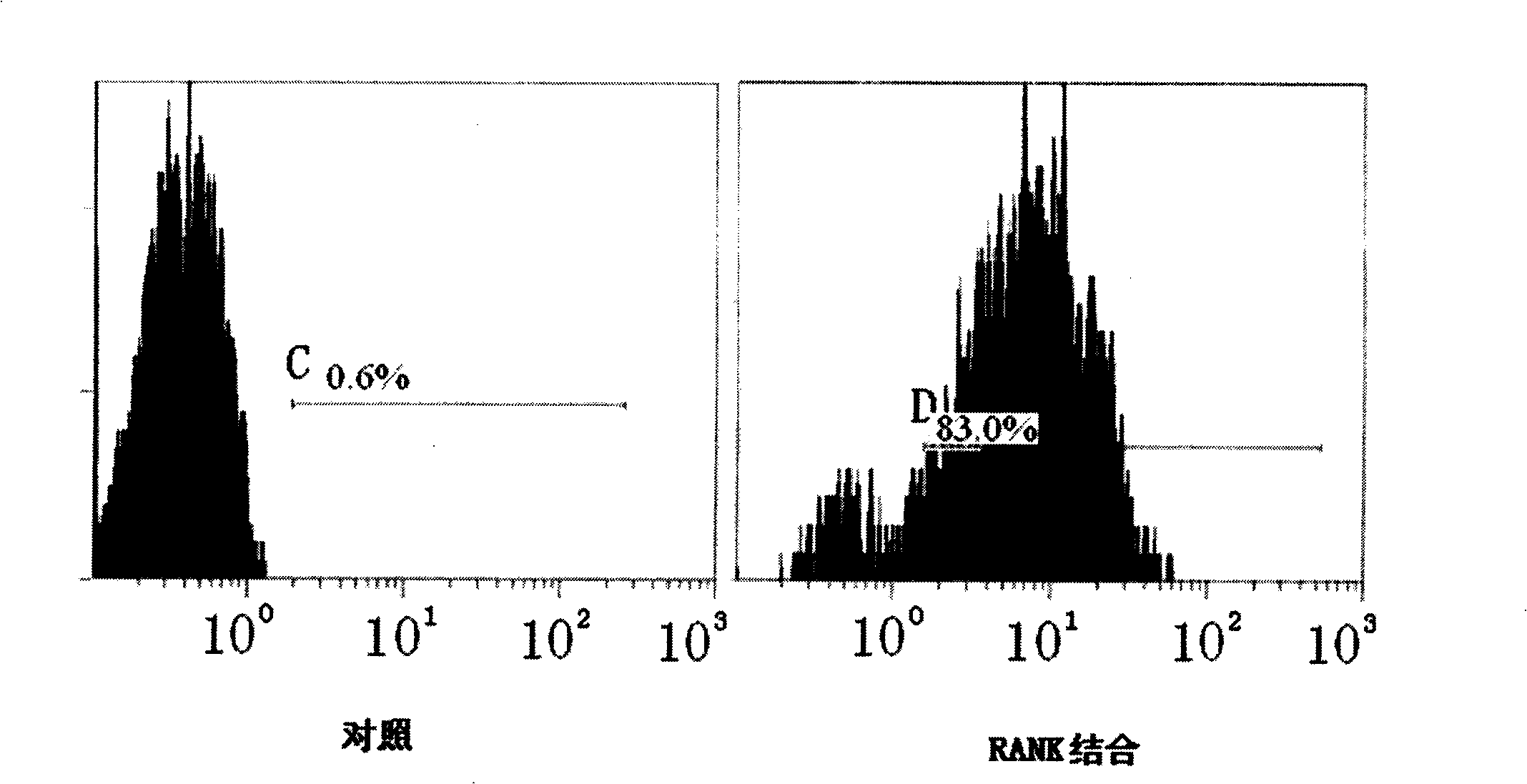
[0053] Mononuclear cells were isolated from human peripheral blood using magnetic bead separation technology. Will 1×10 6 After the monocytes were cultured in MCSF and RANKL medium for 72 hours, they were reacted with 5 micrograms of the purified RAKL-Fc fusion protein in Example 1 for 1 hour at 4° C., and 5 microliters of FITC-labeled anti-human RANKL antibody (CALTAG, CA ), fluorescently labeled cells were detected by flow cytometry. The result is as figure 1 As shown, it shows that the fusion protein RIG expressed in Example 1 can combine with RANK, and THP1 cells are FITC positive. figure 1 Among them, SSC-H is the number of particles in the cell, FSC-H is the cell size, FL2-H is PE, and FL1-H is FITC.
Embodiment 3
[0054] Example 3 Binding experiment of RANKL-Fc fusion protein and FcγRII receptor
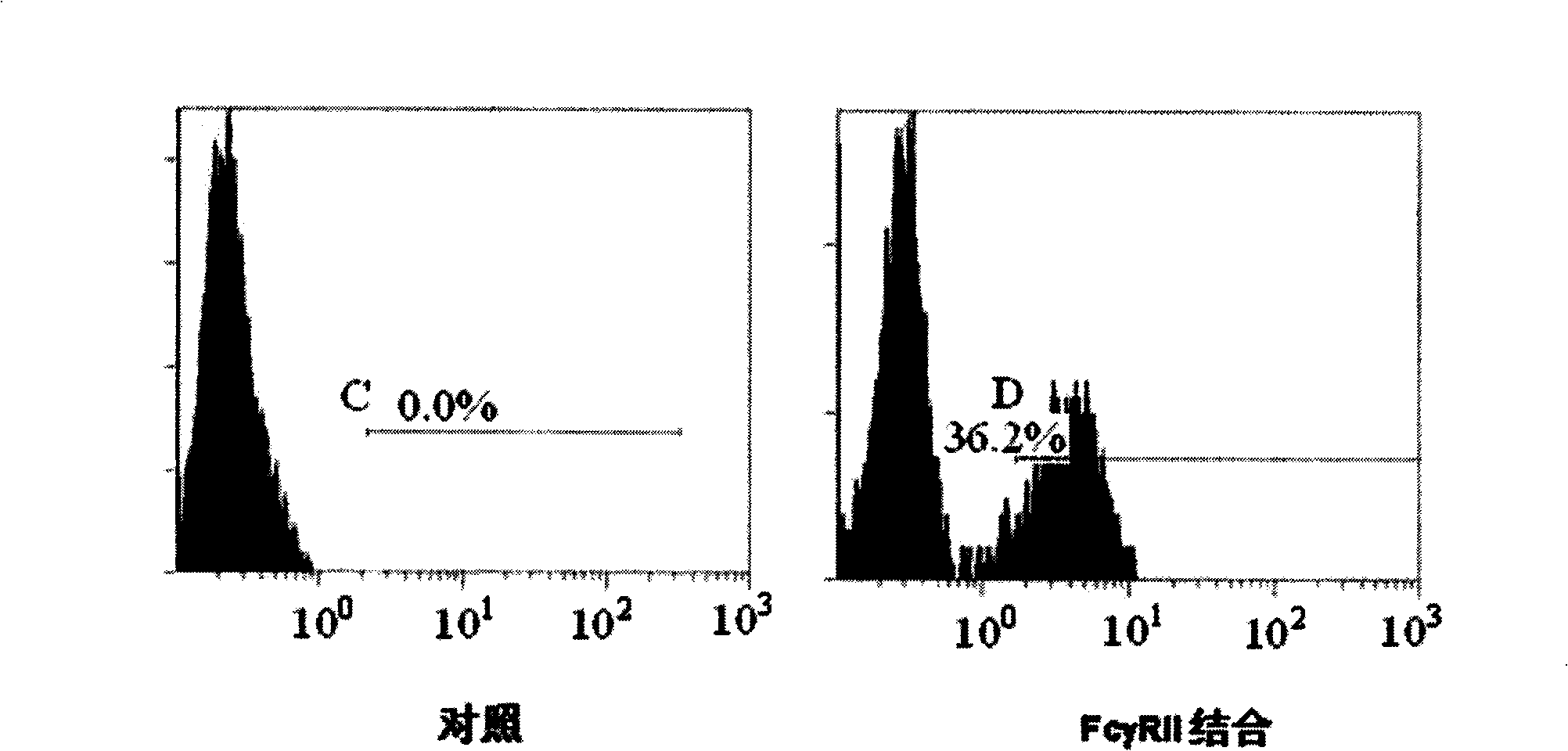
[0055] HMC-1 is a high-expression cell line screened in Example 1, and expresses FcγRII on the cell surface. HMC-1 cells were cultured in DMEM medium containing 10% fetal bovine serum. Will 1×10 6 HMC-1 cells were reacted with 5 micrograms of the RANKL-Fc fusion protein purified in Example 1 at 4°C for 1 hour, and 5 microliters of FITC-labeled anti-human IgG FITC-labeled antibody (CALTAG, CA) was added, and detected by flow cytometry cell. The result is as figure 2 As shown, it shows that the fusion protein RIG expressed in Example 1 can bind to the FcγRII receptor, and HMC-1 cells are FITC positive. figure 2 Among them, SSC-H is the number of particles in the cell, FSC-H is the cell size, FL2-H is PE, and FL1-H is FITC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com