Process for preparation of esters solvent
An ester solvent and a technology for esterification reaction are applied in the field of high boiling point ester solvents and their preparation, which can solve the problems of low metal content and acid content, and achieve the effects of efficient preparation and simple purification operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
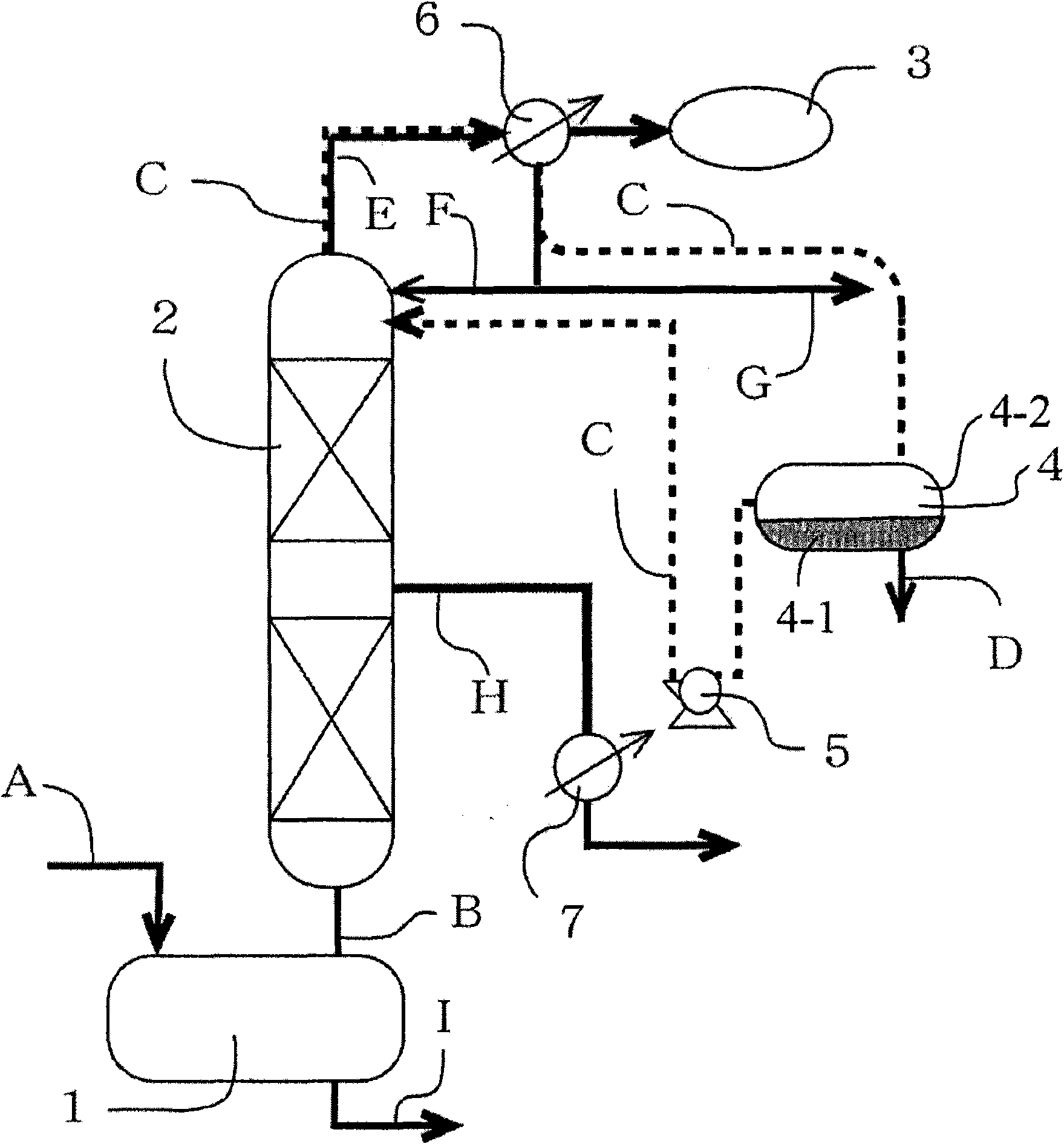
[0030] The preparation method of ester solvent
[0031] Hereinafter, the preparation method of the ester solvent of the present invention will be described with reference to the accompanying drawings when necessary. figure 1 It is a schematic diagram of an example of a device (batch distillation device) suitable for the preparation method of ester solvent of the present invention. Using this batch distillation apparatus, the reaction and subsequent distillation purification can be efficiently performed. exist figure 1 Among them, symbol 1 represents a distillation pot (hereinafter referred to as a distillation pot) capable of reacting alcohol and acid inside, symbol 2 represents a distillation tower, symbol 3 represents a vacuum unit, symbol 4 represents a decanter, and symbol 4-1 represents a decanter The water layer in the decanter, symbol 4-2 represents the organic layer in the decanter. Reference numeral 5 denotes a pump, and reference numerals 6 and 7 denote heat excha...
Embodiment 1
[0053] 100 parts by weight of cyclohexanol, 66 parts by weight of acetic acid, 2 parts by weight of p-toluenesulfonic acid monohydrate, and 19 parts by weight of isobutyl acetate were put into a still. The pressure at the top of the distillation column was decompressed to 40kPa.A for total reflux. If water is generated in the distillate, the distillate is subjected to liquid-liquid separation in a decanter. The organic layer was refluxed at the top of the tower, and the water layer was discharged from the system. When water is no longer generated, it is considered that the dehydration esterification reaction is completed, and the reaction stock solution is obtained.
[0054] The reaction stock solution was directly used figure 1 equipment for batch distillation. Isobutyl acetate and acetic acid were separated by overhead distillation. At this time, the reflux ratio is designed to be 0.5, the upper limit of the still temperature is 150°C, and the pressure is reduced step by...
Embodiment 2
[0057] 100 parts by weight of 1,3-butanediol, 147 parts by weight of acetic acid, 4 parts by weight of p-toluenesulfonic acid monohydrate, and 28 parts by weight of isobutyl acetate were put into a still. The pressure at the top of the distillation column was decompressed to 40kPa.A for total reflux. If water is generated in the distillate, the distillate is subjected to liquid-liquid separation in a decanter. The organic layer was refluxed at the top of the tower, and the water layer was discharged from the system. When water is no longer generated, it is considered that the dehydration esterification reaction is completed, and the reaction stock solution is obtained.
[0058] The reaction stock solution was directly used figure 1 equipment for batch distillation. Isobutyl acetate and acetic acid were separated by overhead distillation. At this time, the reflux ratio is designed to be 0.5, the upper limit of the still temperature is 150°C, and the pressure is reduced step...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com