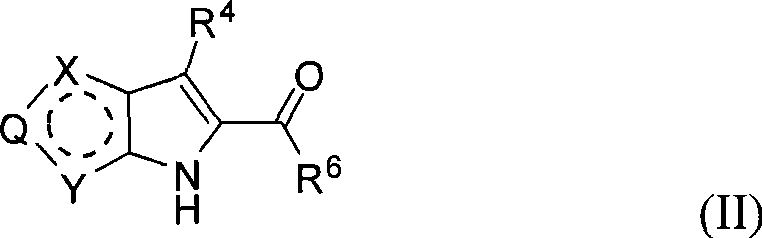
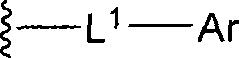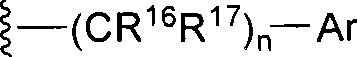Fused heterocyclic inhibitors of D-amino acid oxidase
A compound, the technology of heterocycloalkyl, applied in the field of fused heterocyclic inhibitors of D-amino acid oxidase, can solve the problems of improving or enhancing learning, memory or cognition etc. which are not mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
Synthesis of Fused Thiophenepyrrole Analogs
1.1. Synthesis of intermediate product aldehyde
1.1.a) Synthesis of 4-(4-chlorobenzyl)thiophene-2-carbaldehyde
[0257] Pd(OAc) 2 (144mg, 0.64mmol) and triphenylphosphine (TPP) (136mg, 0.5 2 mmol) solution mixture was weighed into a vial, dissolved in acetonitrile, and transferred into a solution containing 4-chlorobenzyl diethyl phosphate (Org. Lett. 2005, 7, 4875-4878; 3.08g, 11.6mmol), 5-methyl Acylthiophen-3-ylboronic acid (2.0g, 12.8mmol), K 3 PO 4 (2.72g, 12.8mmol) and a stir bar in a 40mL Wheaton vial. Nitrogen was bubbled through the mixture. The vial was tightly sealed, heated to 90 °C, and stirred vigorously for 16 h. The reaction was diluted with water and extracted with dichloromethane (DCM) (3 x 100 mL). The combined extracts were washed with brine, passed through Na 2 SO 4 Dry, filter and concentrate. Purification by flash chromatography (Isco CombiFlash) (0-20% Heptane / EtOAc) gave 4-(4-chlorobenzyl)th...
Embodiment 2
Synthesis of Fused Pyrrole Analogs
2.1. Synthesis of intermediate product aldehyde
2.1.a) Synthesis of 4-phenethyl-furan-2-carbaldehyde
[0357] Under a flow of argon, flush 4-bromo-2-furfural (1.50 g, 8.57 mmol), PdCl 2 (PhCN) 2 (197 mg, 0.514 mmol) and CuI (65.0 mg, 0.343 mmol) as a solid mixture for 1 min. HP (tert-butyl) 3 BF 4 (298 mg, 1.03 mmol) and diisopropylamine (1.80 mL, 12.9 mmol) in dioxane (9 mL) were added to the solid mixture, followed by phenylacetylene (1.13 mL, 10.3 mmol). The reaction was stirred at room temperature under argon atmosphere for 15 h before being filtered through a plug of silica gel with EtOAc. The solution was then concentrated in vacuo and chromatographed on silica gel to give 4-phenylethynyl-furan-2-carbaldehyde as a colorless oil (1.54 g, 92%). R f = 0.35 (1:9 Heptane / EtOAc); 1 H NMR (400MHz, CDCl 3 ) δ ppm 9.68 (d, J = 0.5 Hz, 1H) 7.90 (s, 1H) 7.48-7.55 (m, 2H) 7.35-7.40 (m, 3H) 7.33 (d, J = 0.7 Hz, 1H).
[0358] To...
Embodiment 3
[0446] To a cooled (0 °C) solution of methyl-2-pyrrolecarboxylate (8.00 g, 63.9 mmol) in DMF (320 mL) was added NaH (60% by weight, 5.10 g, 128 mmol). After 20 min, benzyl bromide (11.4 mL, 95.9 mmol) was added and the reaction was allowed to warm to room temperature. Stirring was continued for 2 hours, then saturated NH 4 Quenched with aqueous Cl (0.5 L). The mixture was extracted 3 times with EtOAc and washed with H 2 The combined organic layers were washed with O (3x) and brine, passed over MgSO 4 Dry, filter and concentrate in vacuo to yield a yellow oil. The crude product was chromatographed on silica gel (0 to 10% EtOAc in heptane, 25 min) to give methyl 1-benzyl-1H-pyrrole-2-carboxylate as a colorless oil (7.75 g, 56%) . R f = 0.48 (25:75 Heptane / EtOAc); 1 H NMR (400MHz, CDCl 3 )δ (ppm) 7.28-7.34 (m, 2H) 7.23-7.27 (m, 1H) 7.09-7.13 (m, 2H) 7.01 (dd, J = 4.0, 1.8Hz, 1H) 6.88-6.91 (m, 1H) 6.19 (dd, J=4.0, 2.6 Hz, 1H) 5.57 (s, 2H).
[0447] To a solution of methy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com