Process for preparation of high purity methacrylic acid
A technology of pure methacrylic acid and methacrylic acid, which is applied in the direction of carboxylate preparation, carboxylate preparation, chemical instruments and methods, etc., and can solve the problems such as the decline of methacrylic acid yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
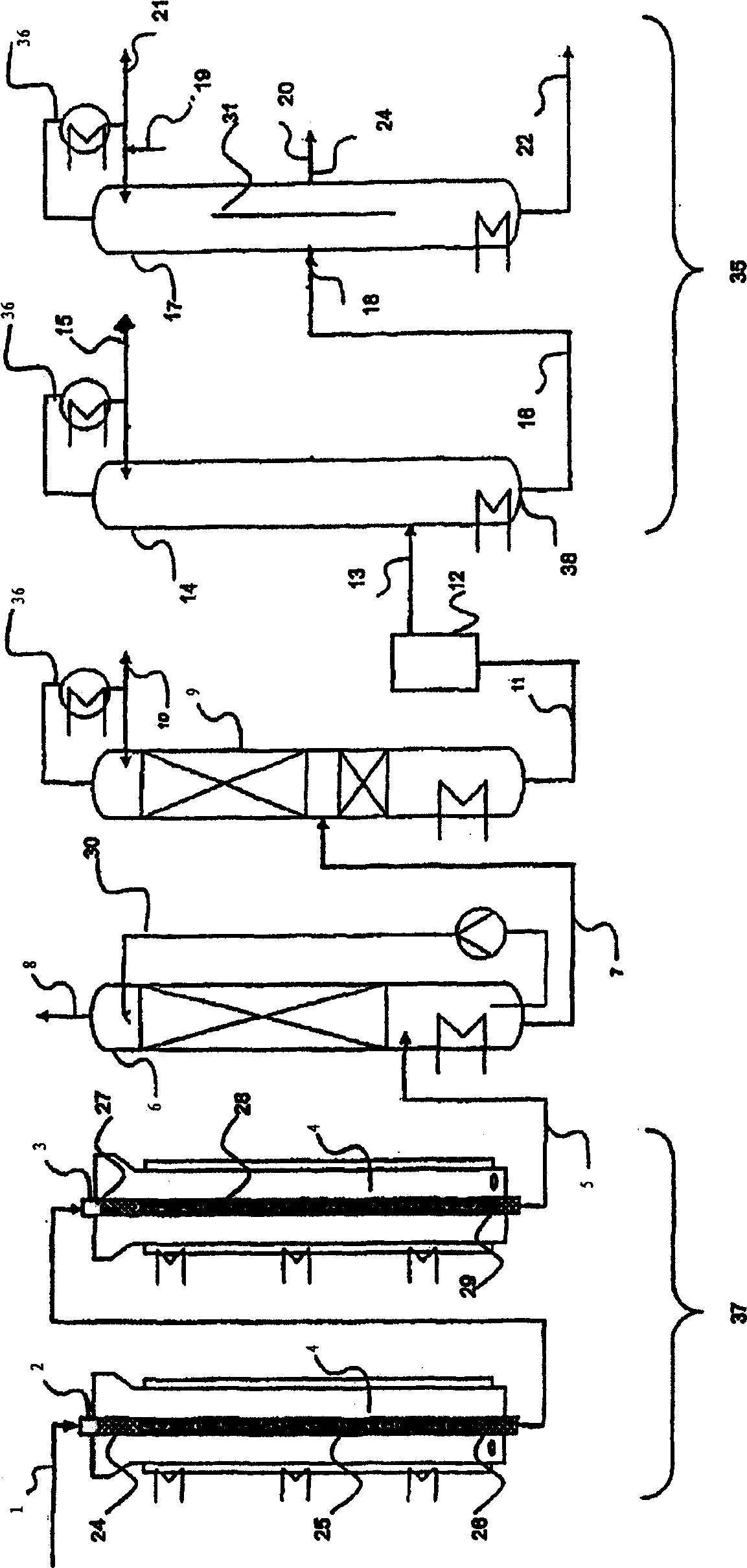
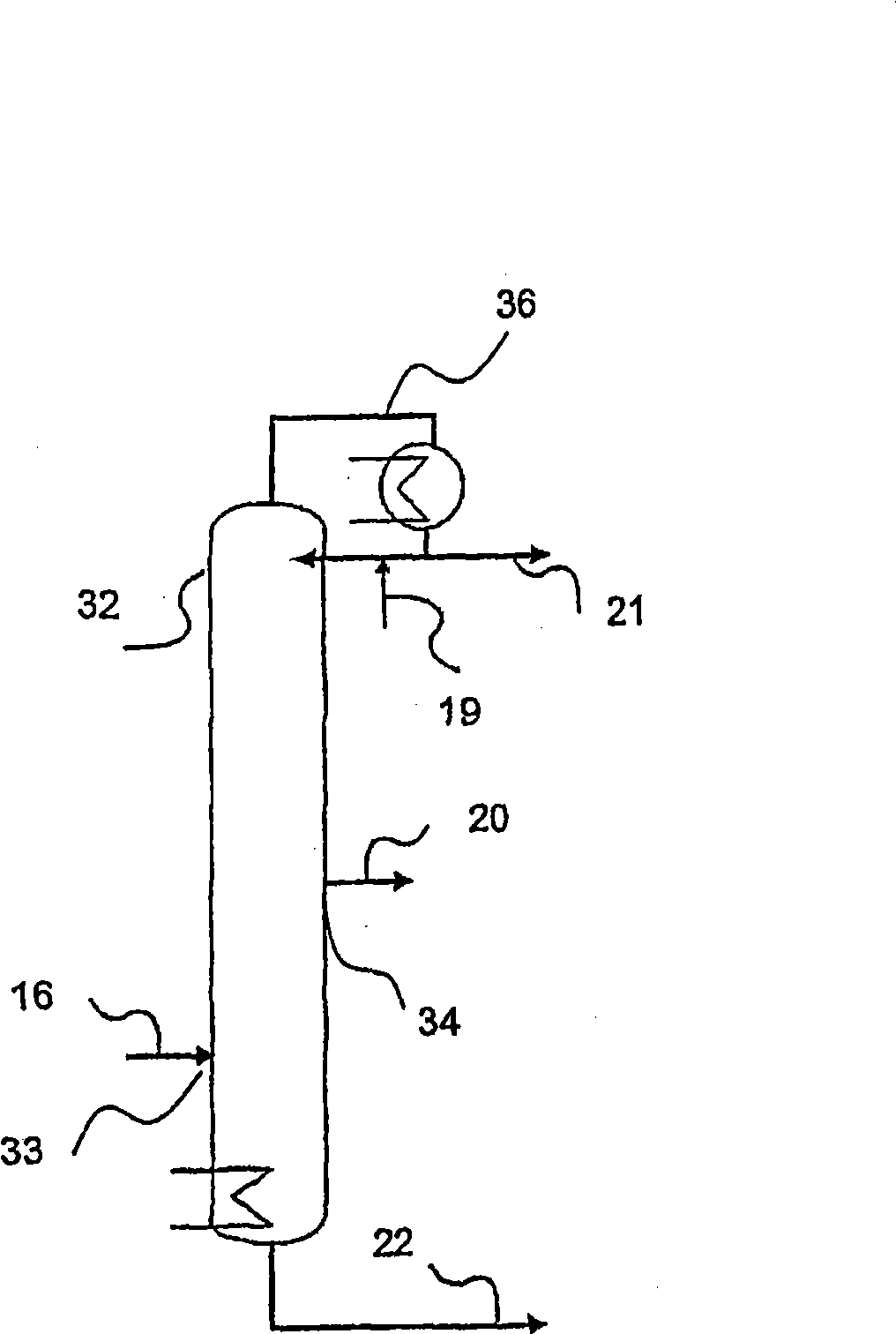
[0122] use figure 1 The device, the difference is that instead of column 17, use figure 2 The column (column 32) serves as the distillation column of the purification unit. The salt bath temperatures of the first and second reaction tubes were 360°C for the first reactor and 300°C for the second reactor, respectively. Isobutene (5 vol.%) was oxidized in two steps with air (85 vol.%) and water (10 vol.%) to form a reaction product containing methacrylic acid in the gas phase. This reaction product is condensed in a quench column to form an aqueous methacrylic acid solution and then separated from low boiling components in a first distillation column. An aqueous methacrylic acid solution having the composition given in Table 1 without the methacrylic acid component (in which the total weight including methacrylic acid adds up to 100 wt. %) was obtained. Methacrylic acid is extracted from aqueous methacrylic acid with n-heptane, as described in EP 0 710 643 A1. The resulting...
Embodiment 2
[0128] use figure 1 The device, and the crude methacrylic acid side inlet and the pure methacrylic acid side outlet at similar heights in the rectification column.
[0129] Methacrylic acid having a composition according to Table 2 was withdrawn continuously at the side outlet for pure methacrylic acid.
Embodiment 3
[0131] This example corresponds to example 1, wherein aminoguanidine bicarbonate (1000 ppm) was added to the reflux of the rectification column. Methacrylic acid having the composition of Table 2 was withdrawn continuously at the top of the column.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com