Fermentation and purification of actinomadura chromoprotein and related species
A technology of actinomycetes madura and chromoproteins, applied in fermentation, methods using bacteria, methods based on microorganisms, etc., can solve the problems of reduced toxicity and increased stability, and achieve reduced toxicity, increased stability, and high efficacy increased effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0188] It is understood and anticipated that variations on the principles of the invention may be effected by persons skilled in the art and that such modifications are intended to be included within the scope of the invention.
[0189] The following examples of the invention are set forth to further illustrate the invention and should not be construed as limiting the invention in any way.
example 1
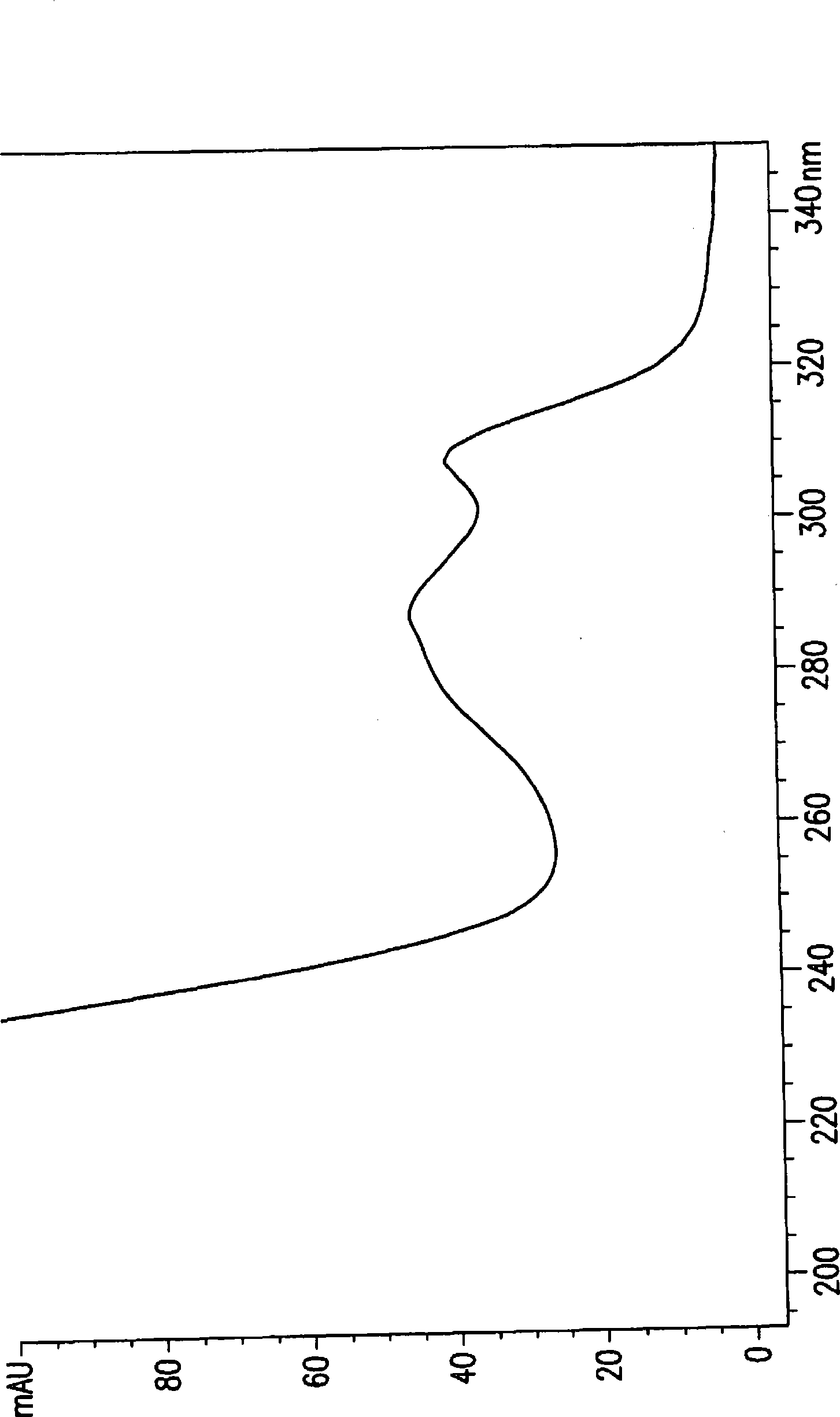
[0191] Separation and characterization of chromoprotein and apoprotein
[0192] Example 1 - Seed Culture
[0193] Actinomyces madurai 21G792 was preserved as frozen whole cells (frozen vegetative mycelium, FVM) prepared from cells grown in ATCC medium 172 for 72 hours (dextrose 1%, soluble starch 2%, yeast extract 0.5%, and N-Z amine type A 0.5%, CaCO 3 0.1% pH 7.3). Glycerol was added to 20% and cells were frozen at -150°C.
[0194] A seed medium containing pH 6.9 was prepared containing: 1.0% dextrose, 2.0% soluble starch, 0.5% yeast extract, 0.5% N-Z amine type A (Sheffield), and 0.1% CaCO 3 . In a 25mm x 150mm glass culture tube, inoculate 7ml of seed medium and two glass beads. Sufficient inoculum from the agar culture was used to provide mixed seeds after 72 hours of incubation. The primary seed tubes were incubated for 72 hours at 28°C, 250 rpm using a gyro spinner flask with a 2 inch eccentricity. Primary seeds (approximately 14% inoculum) were then used to in...
example 2
[0196] Example 2 - Fermentation
[0197] Prepare a ferment production medium at pH 6.9 containing: 2.0% sucrose, 0.5% molasses, 0.5% CaCO 3 , 0.2% peptone, 0.002% magnesium sulfate-7H 2 O, 0.001% ferrous sulfate-7H 2 O, 0.05% sodium bromide, and 0.2% sodium acetate. Sixty 250ml Erlenmeyer flasks each with 50ml of ferment production medium were inoculated with 2ml (4.0%) of secondary seed ferment and gyroscoped at 28°C, 250rpm (2" eccentric distance) Cultivation is carried out.The fermentation is then continued for about 72 to 96 hours and harvested for further processing.
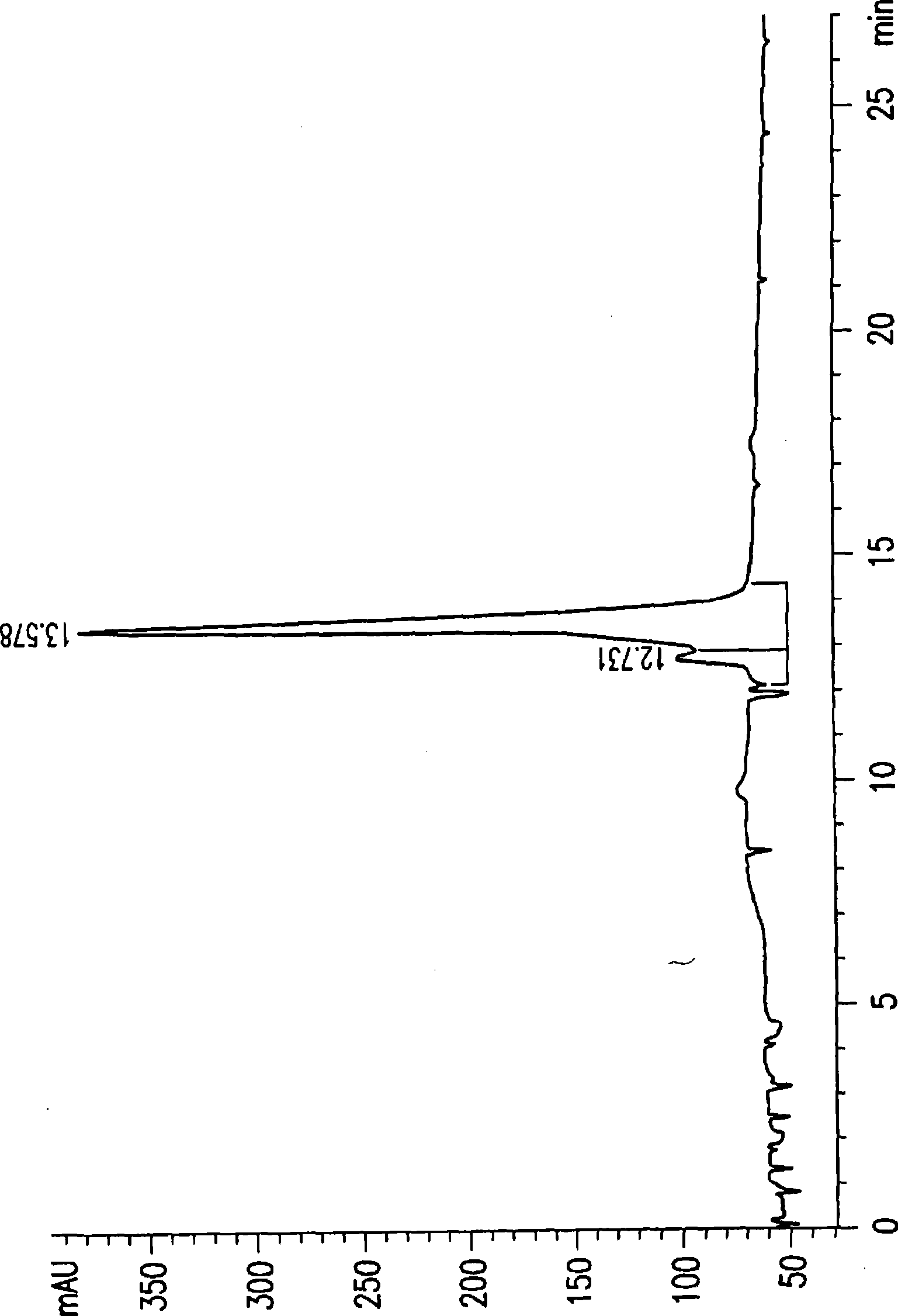
[0198] The combined whole broth (60x50ml) was centrifuged at 3800 rpm for 30 minutes. The supernatant was then lyophilized and the residual powder was suspended in a small volume (eg, 300 ml) of H 2 O middle. After centrifugation, the brown solution was then loaded at 4 °C in the dark with 2 6L Sephadex G75 glass column in O. Fractions collected were each 40 ml and tested in a Biochemical Induction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com