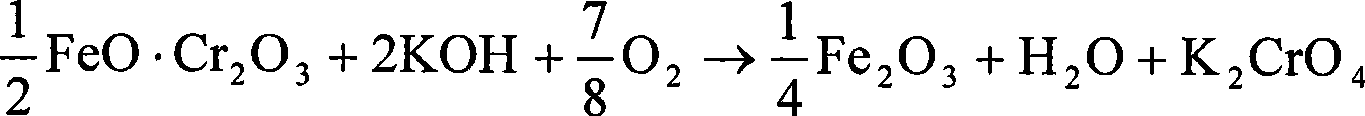Clean production method for preparing potassium chromate from chromic iron
A technology of potassium chromate and chromite, applied in the direction of chromate/dichromate, etc., can solve problems such as reducing KOH, and achieve the effect of reducing corrosion, improving industrial operability, and reducing the requirements for corrosion resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] KOH-KNO recycled back to the reactor 3 -H 2 O medium, heat up to 300°C after adding KOH, add >95% chromite with a particle size of less than 200 mesh, add potassium peroxide, and react for 6 hours under complete mixing conditions, wherein the weight ratio of KOH to ore is 2: 1. KNO 3 The weight ratio with ore is 0.5:1. Finally get KOH-KNO 3 -H 2 The mixed reaction product of O medium, chromium salt and iron slag, the conversion rate of chromium is 99%. The mixed reaction product is leached with water, and the temperature after leaching is 90°C; the lye containing potassium nitrate is first separated from the leaching slurry, and then the iron slag is separated from the potassium chromate leaching solution; the impurity content to be removed is 1.5 times Calcium oxide was added into the potassium chromate solution, and the precipitate was removed by filtration after the reaction was completed; the pure potassium chromate solution was evaporated and crystallized, and...
Embodiment 2
[0030] KOH-KNO recycled back to the reactor 3 -H 2O medium, heat up to 330°C after adding KOH, add >95% chromite with a particle size of less than 200 mesh, feed oxygen, and react for 4 hours under the condition of complete mixing, wherein the weight ratio of KOH to ore is 1.5: 1. KNO 3 The weight ratio with ore is 1:1. Finally get KOH-KNO 3 -H 2 The mixed reaction product of O medium, chromium salt and iron slag, the conversion rate of chromium is 99%. The mixed reaction product is leached with water, and the temperature after leaching is 150°C; the lye containing potassium nitrate is first separated from the leaching slurry, and then the iron slag is separated from the potassium chromate leaching solution; the impurity content to be removed is 1.5 times Calcium oxide is added into the potassium chromate solution, and the precipitate is removed by filtration after the reaction is completed; the pure potassium chromate solution is evaporated and crystallized, and the crys...
Embodiment 3
[0032] KOH-KNO recycled back to the reactor 3 -H 2 O medium, heat up to 390°C after adding KOH, add >95% chromite with a particle size of less than 200 mesh, feed air, and react for 1 hour under the condition of complete mixing, wherein the weight ratio of KOH to ore is 1: 1. KNO 3 The weight ratio with ore is 1.5:1. Finally get KOH-KNO 3 -H 2 The mixed reaction product of O medium, chromium salt and iron slag has a chromium conversion rate of 99%, and the chromium content in the slag is 0.06%. The mixed reaction product is leached with water, and the temperature after leaching is 90°C; the lye containing potassium nitrate is first separated from the leaching slurry, and then the iron slag is separated from the potassium chromate leaching solution; the impurity content to be removed is 1.5 times Calcium oxide was added into the potassium chromate solution, and the precipitate was removed by filtration after the reaction was completed; the potassium chromate solution was e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com