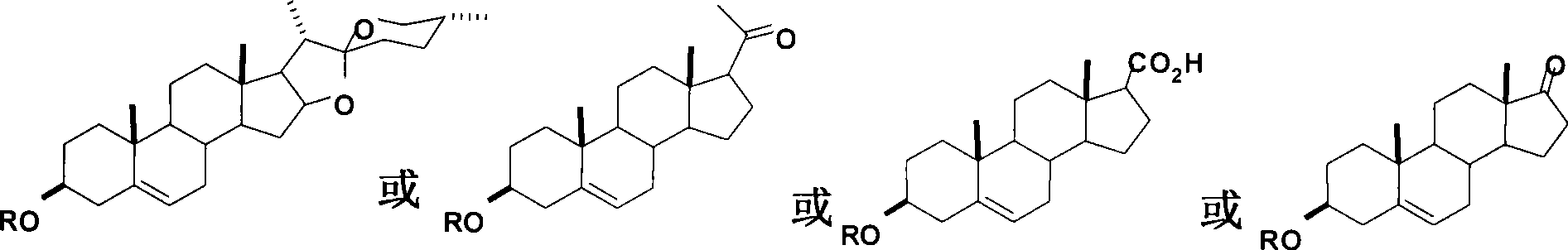
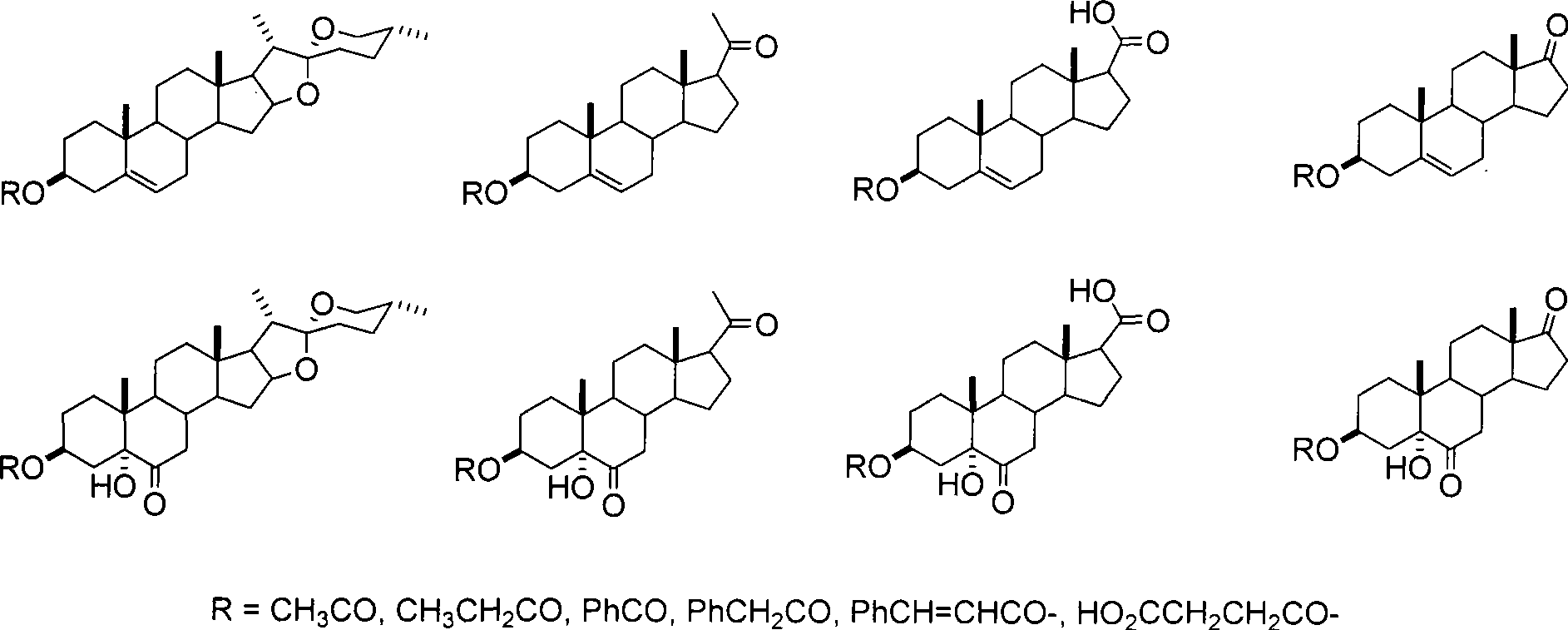
Steroid compounds with endothelin receptor A antagonistic activity and intermediate thereof
A compound and steroid technology, applied in the field of new steroid compounds and their intermediates, can solve the problems of long synthetic routes and complicated processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013]
[0014] Synthesis of Acetyl Diosgenin:
[0015] Diosgenin (5.00 g, 12.1 mmol) was dissolved in 60 mL of pyridine, 7.2 mL of acetic anhydride and 0.5 g of DMAP were added with stirring, and stirring was continued at room temperature for 4.5 hours. Pour the reaction solution into 300mL of ice water under stirring, continue to stir for 10 minutes and then filter with suction, wash repeatedly with appropriate amount of water to remove pyridine as much as possible, and dry to constant weight under infrared light to obtain 5.40g of white solid (R f =0.70, ethyl acetate:n-hexane=1:4), the yield was 98%.
[0016] ESI-MS(+): m / z 479[M+Na] + .
[0017] 3β-Acetoxy-5,6-epoxydiosgenin
[0018] Acetyldiosgenin (2.74g, 6mmol) was dissolved in 30mL of chloroform, m-CPBA (1.58g, 9mmol) was added with stirring, and stirring was continued at room temperature for 5 hours, during which the reaction system changed from clear to white turbid. After the reaction stopped, 30 mL of chlor...
Embodiment 2
[0033]
[0034] Acetyl dehydroepiandrosterone
[0035] Method 1: Dissolve dehydroepiandrosterone (1.44g, 5mmol) in 15mL of dichloromethane, add 1.2mL of pyridine, 1mL of acetic anhydride and 60mg of N,N'-dimethylaminopyridine (DMAP) under stirring, Stirring was continued for 12 hours. Wash with dilute hydrochloric acid (2 times the equivalent of pyridine) under ice-cooling conditions. The organic phase was washed successively with 15 mL of water, 15 mL of 10% sodium bicarbonate solution, and 15 mL of water, and the obtained aqueous phase was washed with 5 mL of dichloromethane, and the organic phases were combined. After the solvent was removed by rotary evaporation, the obtained white solid was washed with n-hexane to obtain 1.22 g of white solid, with a yield of 74.0%.
[0036] Method 2: Dissolve dehydroepiandrosterone (2.88 g, 10 mmol) in 40 mL of pyridine, add 6 mL of acetic anhydride and 120 mg of DMAP under stirring, and continue stirring at room temperature for 4.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com