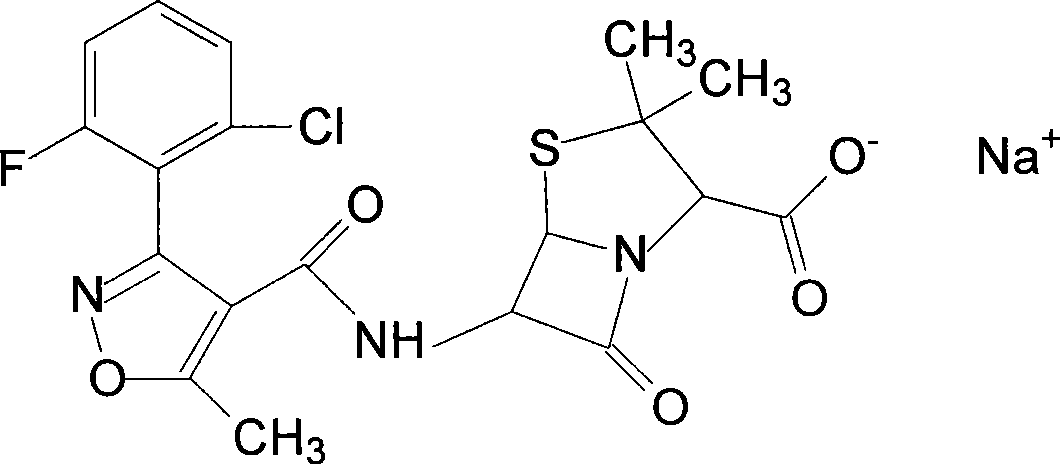
Flucloxacillin sodium compound and preparation thereof
A technology of flucloxacillin sodium and flucloxacillin, which is applied in the field of flucloxacillin sodium compound and its preparation, can solve problems such as reducing product yield, achieve the effects of improving purity, ensuring drug safety, and stabilizing product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] i, the preparation of flucloxacillin N'N-dibenzylethylenediamine salt
[0026] Dissolve 951.7 g of 2 mol of flucloxacillin sodium in 15 L of water, adjust the pH value to 3.0 with hydrochloric acid, then add 8.5 L of methanol, and then add 1576.6 g of 4.4 mol N'N-dibenzylethylenediamine diacetate, at 20 Mix and stir the reaction at ~25°C for 3 hours to produce a white solid. Cool the reaction mixture to 10-15°C, filter, wash the white solid three times with water, then wash three times with methanol, and dry it in vacuum at 30-35°C to obtain 1136.8 g of solid. The rate is 98.8%. The melting range of the crystal is 192-198°C. The salt is washed with water and methanol to obtain a product with a melting point of 195-198°C. Analysis shows that the crystal is N'N-dibenzylethylenediamine: flucloxacillin acid is 1;1 mixture.
[0027] ii, the preparation of flucloxacillin sodium salt
[0028] Suspend 1100 g of flucloxacillin N'N-dibenzylethylenediamine salt crystals in 11 L...
Embodiment 2
[0030] i, the preparation of flucloxacillin N'N-dibenzylethylenediamine salt
[0031] Dissolve 1427.4 g of 3 mol of flucloxacillin sodium in 25 L of water, adjust the pH value to 4.0 with acetic acid, then add 9.5 L of acetone, and then add 537.5 g of 1.5 mol of N'N-dibenzylethylenediamine diacetate, at 20 Mix and stir at ~25°C for 5 hours to produce a white solid. Cool the reaction mixture to 10-15°C, filter, wash the white solid three times with water, then wash three times with acetone, and dry under vacuum at 30-35°C to obtain 1536.8 g of solid. The rate is 99.1%. The melting range of the crystal is 192-198°C. The salt is washed with water and then acetone to obtain a product with a melting point of 195-198°C. Analysis shows that the crystal is N'N-dibenzylethylenediamine: flucloxacillin acid is 1.2:1 mixture.
[0032] ii, the preparation of flucloxacillin sodium salt
[0033] Suspend 1200g of flucloxacillin N'N-dibenzylethylenediamine salt crystals in 6L of ethanol, wh...
Embodiment 3
[0035] i, the preparation of flucloxacillin N'N-dibenzylethylenediamine salt
[0036] Dissolve 475.8 g of 1 mol of flucloxacillin sodium in 5 L of water, adjust the pH value to 4.0 to 5.0 with hydrochloric acid, then add 4.5 L of acetonitrile, and then add 4 mol of N'N-dibenzylethylenediamine diacetate 1433 g, at 20 ~ 25 ° C mixed and stirred for 4 hours to produce a white solid, the reaction mixture was cooled to 10 ~ 15 ° C, filtered, the white solid was washed three times with water, then washed three times with acetonitrile, 30 ~ 35 ° C vacuum drying to obtain 566.8 g of solids, product The rate is 98.7%. The melting range of the crystal is 192-198°C. Wash the salt with water and then acetonitrile to obtain a product with a melting point of 195-198°C. Analysis shows that the crystal is N'N-dibenzylethylenediamine: flucloxacillin acid is 1:1.1 mixture.
[0037] ii, the preparation of flucloxacillin sodium salt
[0038] Suspend 500 g of flucloxacillin N'N-dibenzylethylene...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com