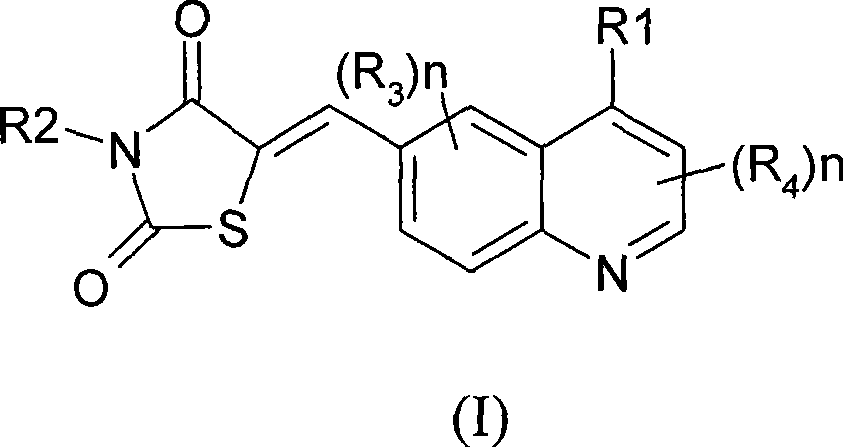
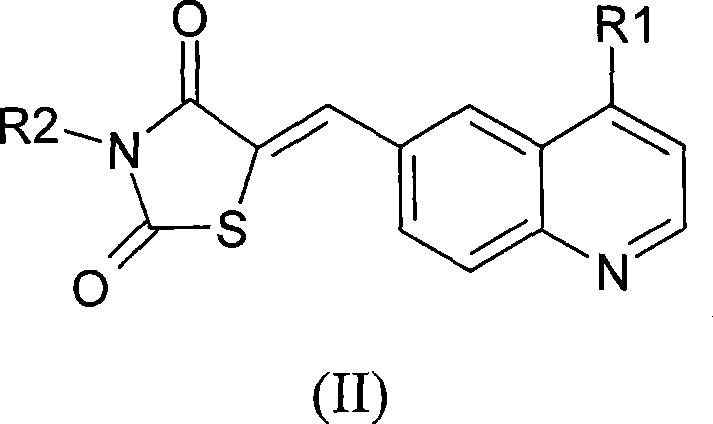
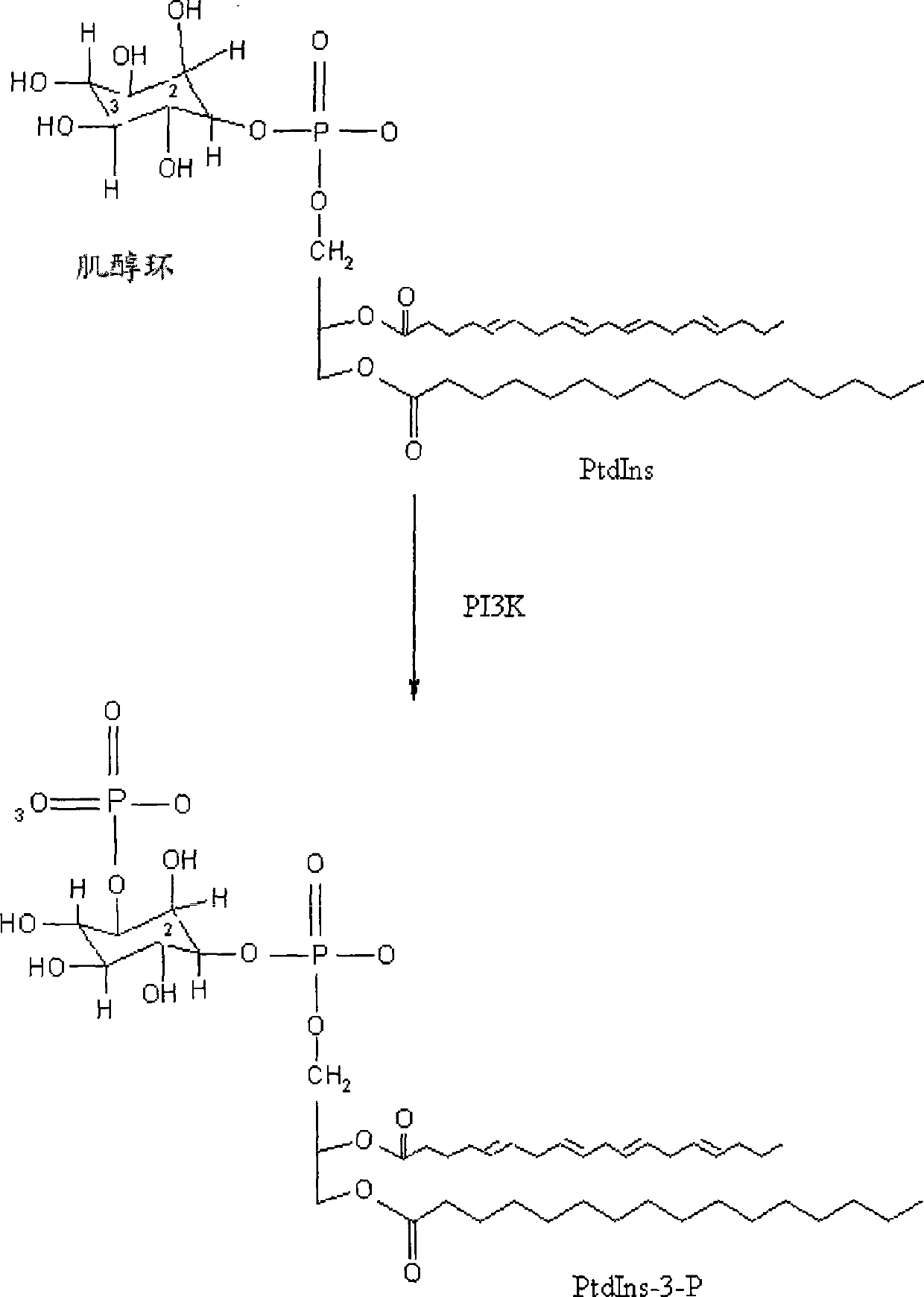
Thiazolidinedione derivatives as PI3 kinase inhibitors
A thiazolidine, dione technology, applied in the field of thiazolidinedione derivatives, can solve problems such as expression restriction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0238] Example 1: (5Z)-5-{[4-(4-pyridyl)-6-quinolyl]methylidene}-1,3-thiazolidine-2,4-dione
[0239]
[0240] a) 4-chloro-6-vinylquinoline
[0241] 6-bromo-4-chloroquinoline (6.52 g, 26.88 mmol; see J. Med. Chem., 21, 268 (1978)), tributyl (vinyl) tin (8.95 g, 28.22 mmol) and tetra( A mixture of triphenylphosphine)palladium(0) (0.62 g, 0.54 mmol) in 1,4-dioxane (150 mL) was refluxed for 2.0 hours, cooled to room temperature, and concentrated in vacuo. The residue was purified by flash silica gel chromatography (0-4% MeOH:CH 2 Cl 2 ) to afford the title compound (5.1 g) as a pale yellow solid. MS(ES)+m / e 190[M+H] + . The above material was used directly in the next step.
[0242] b) 4-Chloro-6-quinolinecarbaldehyde
[0243] 4-Chloro-6-vinylquinoline (5.1g, 26.88mmol), 2,6-lutidine (5.76g, 53.75mmol), (meta)sodium periodate (22.99g, 107.51mmol) and Osmium tetroxide (5.48g, 2.5% tert-butanol solution, 0.538mmol) in 1,4-dioxane: H 2 The mixture in O (350 mL, 3:1 mixtur...
Embodiment 2
[0248] Example 2: (5Z)-5-{[4-(3-pyridyl)-6-quinolyl]methine}-1,3-thiazolidine-2,4-dione
[0249]
[0250] Following the procedure used for the preparation of Example 1, the title compound was prepared by using 3-pyridylboronic acid in place of 4-pyridylboronic acid. MS(ES)+m / e 334[M+H] + .
Embodiment 3
[0251] Example 3: (5Z)-3-methyl-5-{[4-(4-pyridyl)-6-quinolyl]methine}-1,3-thiazolidine-2,4-dione
[0252]
[0253] Following the procedure used for the preparation of Example 1, the title compound was prepared by using 3-methyl-1,3-thiazolidine-2,4-dione in place of 1,3-thiazolidine-2,4-dione to give yellow solid. MS(ES)+m / e 348[M+H] + .
[0254] Alternatively, some of the examples were prepared by palladium-catalyzed Suzuki (or Stille) coupling of heteroarylboronic acids (or heteroarylstannanes) with 4-iodo-6-quinolinecarbaldehyde (Scheme II). The iodide intermediate was prepared by treating the corresponding chloride with 4N HCl followed by sodium iodide. The heteroaryl aldehyde was converted into the title compound following the same procedure as in Preparation Example 1.
[0255] Flowchart II:
[0256]
[0257] Conditions: a) 4N HCl in dioxane, room temperature, 5 minutes; then NaI, 105°C, 18 hours; b) heteroaryl(R)boronic acid, Pd(PPh3) 4 , 2M K 2 CO 3 , dio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com