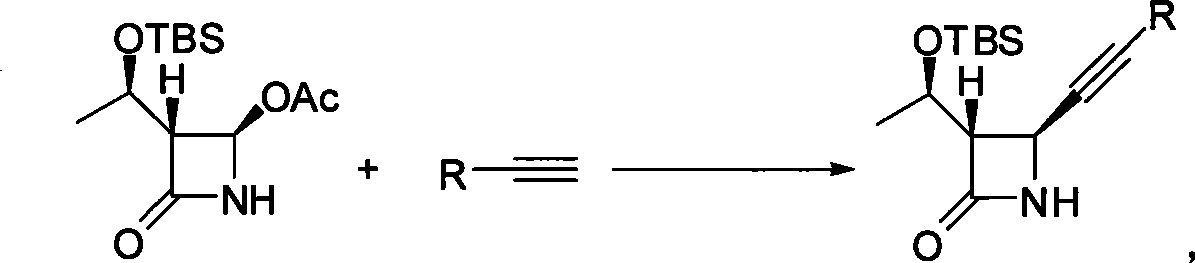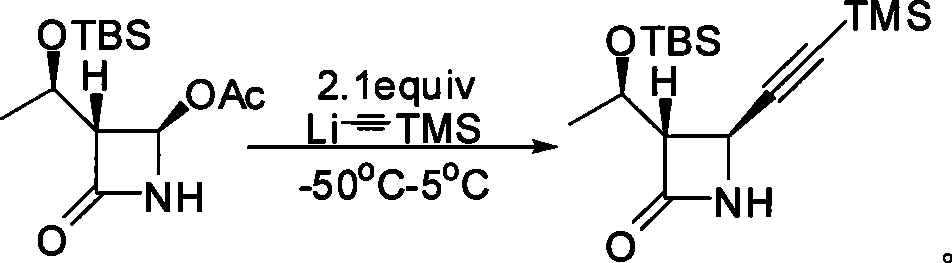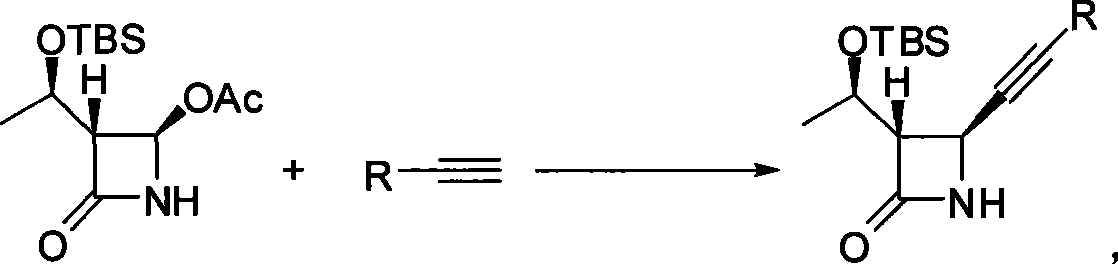Method for preparing 4-alkynyl azetidin-2-one
A technology of azetidinine and alkynyl nitrogen, which is applied in the field of preparation of 4-alkynylazetidin-2-one, can solve the problems of complex operation and unfavorable industrialization, and achieve cheap and easy-to-obtain raw materials, simple route, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of Example 1 (3S, 4S)-3-((R)-1-(tert-butyldimethylsilyloxy) ethyl)-4-phenylethynyl azetidin-2-one
[0028] Zinc trifluoromethanesulfonate (435 mg, 1.2 mmol) was added to 4 mL of toluene, and triethylamine (418 μL, 3.0 mmol) was added, and stirred at 25° C. for 10 minutes. Phenylacetylene (1.2 mmol) was added to the system. Then the mixture was stirred at 40°C for 1 hour, and (2R,3R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl-4-acetylazetidin-2 was added - Ketone (287mg, 1mmol). After reacting for 6 hours, cool to room temperature, add water (8mL) to quench, and extract with ethyl acetate (10mL×2 times). The organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure. After treatment, 240 mg of (3S, 4S)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-4-phenylethynylazetidin-2-one was obtained, producing The rate is 73%.IR: 3.50, 3087, 2955, 2893, 2857, 1763, 1719, 1338, 1137cm -1 ; 1 H NMR (300MHz, CDCl 3 )δ 7.42-7...
Embodiment 2
[0029] Example 2 Synthesis of (3S, 4S)-3-((R)-1-(tert-butyldimethylsilyloxy) ethyl)-4-phenylethynyl azetidin-2-one
[0030] Zinc chloride (163mg, 1.2mmol) was added to 4mL of benzene, then triethylamine (418μL, 3.0mmol) was added, and stirred at 0°C for 10 minutes. Phenylacetylene (2 mmol) was added to the system. Then the mixture was stirred at 0°C for 1 hour, and (2R,3R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl-4-acetylazetidin-2 was added - Ketone (1mmol). After reacting for 6 hours, cool to room temperature, add water (8mL) to quench, and extract with ethyl acetate (10mL × 2 times). Combine the organic phases, dry them over anhydrous sodium sulfate and concentrate under reduced pressure. Aftertreatment Obtained (3S, 4S)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-4-phenylethynylazetidin-2-one 160mg, the yield was 48%.
Embodiment 3
[0031] Example 3 Synthesis of (3S, 4S)-3-((R)-1-(tert-butyldimethylsilyloxy) ethyl)-4-phenylethynyl azetidin-2-one
[0032]Zinc bromide (450mg, 2mmol) was added to 4mL of tetrahydrofuran, and then pyridine (2.0mmol) was added, and stirred at 65°C for 10 minutes. Phenylacetylene (1 mmol) was added to the system. Then the mixture was stirred at 65°C for 2 hours, and (2R,3R)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl-4-acetylazetidin-2 was added - Ketone (1.2mmol). After reacting for 6 hours, cool to room temperature, add water (8mL) to quench, and extract with ethyl acetate (10mL × 2 times). The organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure. After Treatment afforded (3S,4S)-3-((R)-1-(tert-butyldimethylsilyloxy)ethyl)-4-phenylethynylazetidin-2-one 100 mg, yield 30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com