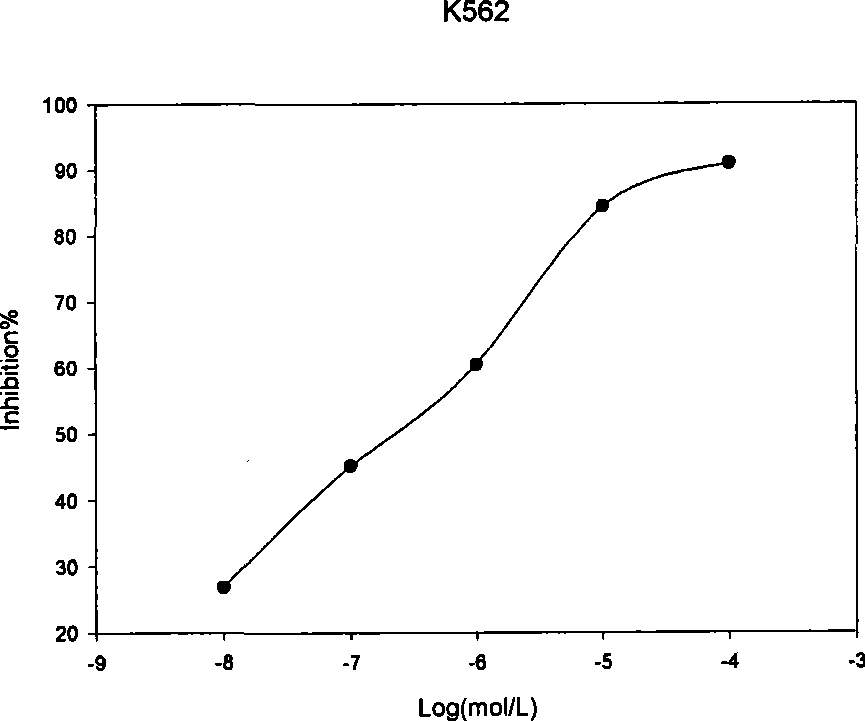
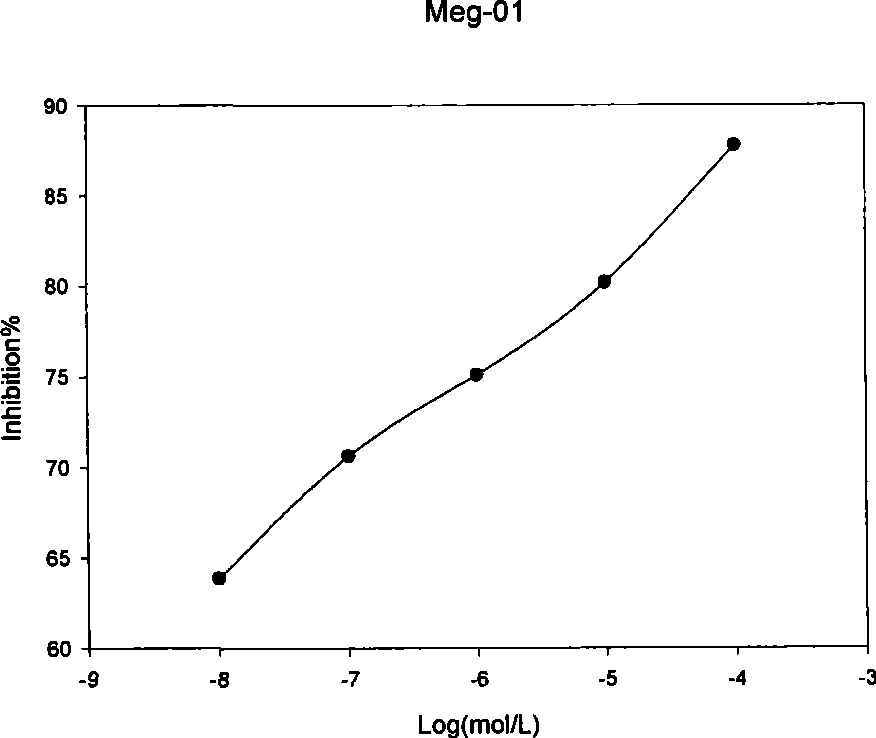
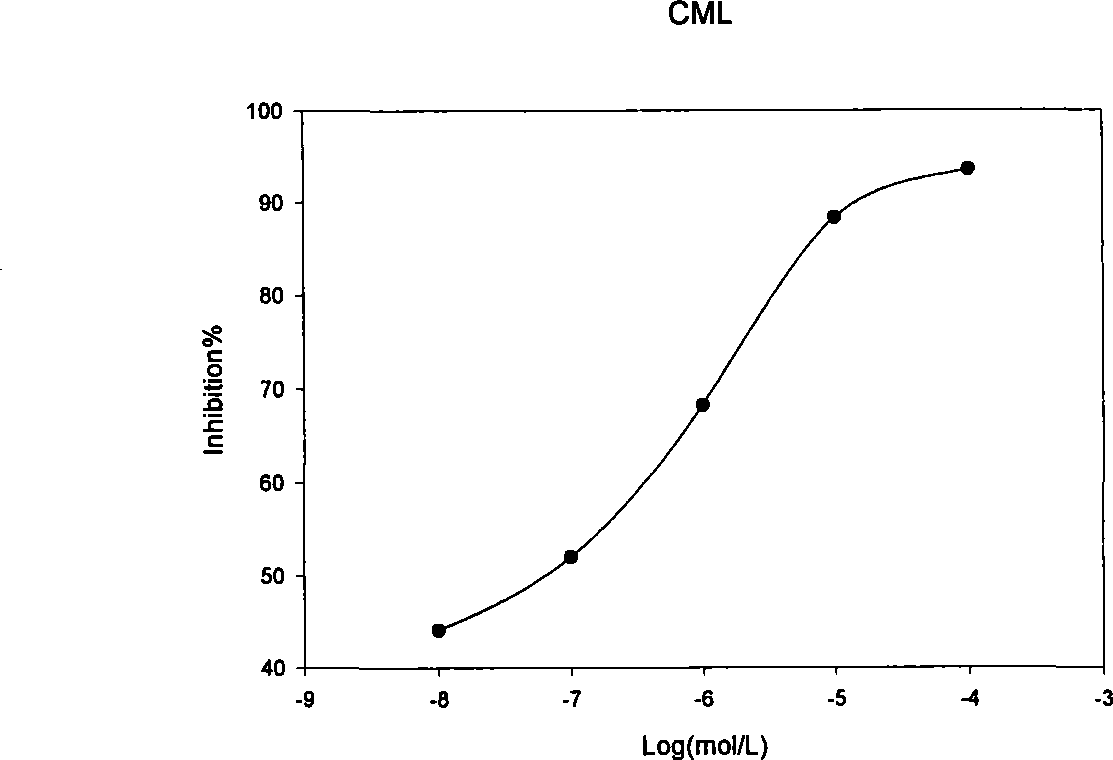
Specificity inhibitor of leukaemia bcr/abl fusion gene mRNA
A leukemia and methyl technology, applied in the field of leukemia gene targeted therapeutic agents, can solve the problems of poor stability, limited development and inhibition, and achieve the effect of simple and feasible synthesis method and overcoming drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of 2β-(4'-methyl-1'-piperazinyl)-3α-hydroxyl-5α-steroid-17-ketone caffeate (V) of the present invention
[0025] (1) Prepare 5α-steroid-2-en-17-one (II) from epiandrosterone (I)
[0026] Dissolve 4g (14mmol) of epiandrosterone (I) in 20ml of anhydrous pyridine, add 4.6g (24mmol) of p-toluenesulfonyl chloride, stir and react for 3h, then reflux for 4h, and recover pyridine to obtain a semi-solid. Add aqueous solution to precipitate a solid, recrystallize after filtration to obtain white crystals, the yield is about 60%, mp.104-105°C, IR(KBr)cm -1 3010 (C=CH), 1735 (C=O), 1650 (C=C).
[0027] (2) Preparation of 2α, 3α-epoxy-5α-steroid-17-one (III)
[0028] 17ml of 8% peracetic acid, 14ml of water and 1.7g of sodium acetate were mixed, and the chloroform solution of II (1.7g dissolved in 13ml of chloroform) was added dropwise. Stir the reaction for 17 hours, separate the organic layer, wash until neutral, concentrate the solid, and get white cryst...
Embodiment 2
[0033] Embodiment 2 Preparation of oral tablet of compound salt of the present invention
[0034] Each tablet contains 3 mg of the compound salt of the present invention, and the tablet is composed as follows:
[0035] Ingredients mg / tablet
[0036] Compound V 3.0
[0037] Microcrystalline cellulose 120.0
[0038] Starch 80.0
[0040] Mix the above ingredients (except magnesium stearate), pass through a 120-mesh sieve, add warm distilled water and stir, then granulate, dry, add magnesium stearate, mix evenly, and tablet to obtain.
Embodiment 3
[0041] Example 3 Preparation of Oral Capsules of Compound Salts of the Present Invention
[0042] Each capsule contains 3 mg of compound salt of the present invention, and the capsules are composed as follows:
[0043] Composition mg / capsule
[0044] Compound V 3.0
[0045] Microcrystalline cellulose 140.0
[0046] Hydroxypropyl cellulose 60.0
[0047] Mix the above ingredients, pass through a 100-mesh sieve, add warm distilled water and stir to make a soft material, pass through a sieve and granulate, dry and fill into capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com