Human-derived anti-human tissue factor Fab and preparation method thereof
A technology of tissue factor and human antibody, which is applied in the field of therapeutic drugs for atherosclerosis, anti-human tissue factor Fab fragment, and Fab antibody, which can solve the problems of bleeding, lack of anticoagulant effect, etc., and reduce immunogenicity , the effect of weakening the effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1. Construction of Antibody Library
[0055] Select 300 informed healthy people who have not suffered from infectious diseases such as colds and chronic diseases in the past 2 months, take 5ml of peripheral anticoagulant blood each, separate lymphocytes with Ficoll-Paque, mix them for the construction of antibody library, and extract the total RNA. Use the Gene-Amp RNA PCR Kit to total RNA with Oligo(dT) 16 Reverse transcribe into cDNA, and carry out PCR amplification of immunoglobulin γ, κ, λ chain genes with the upstream and downstream primers (Table 1) of the conserved sequence of the human IgG light and heavy chain variable region.
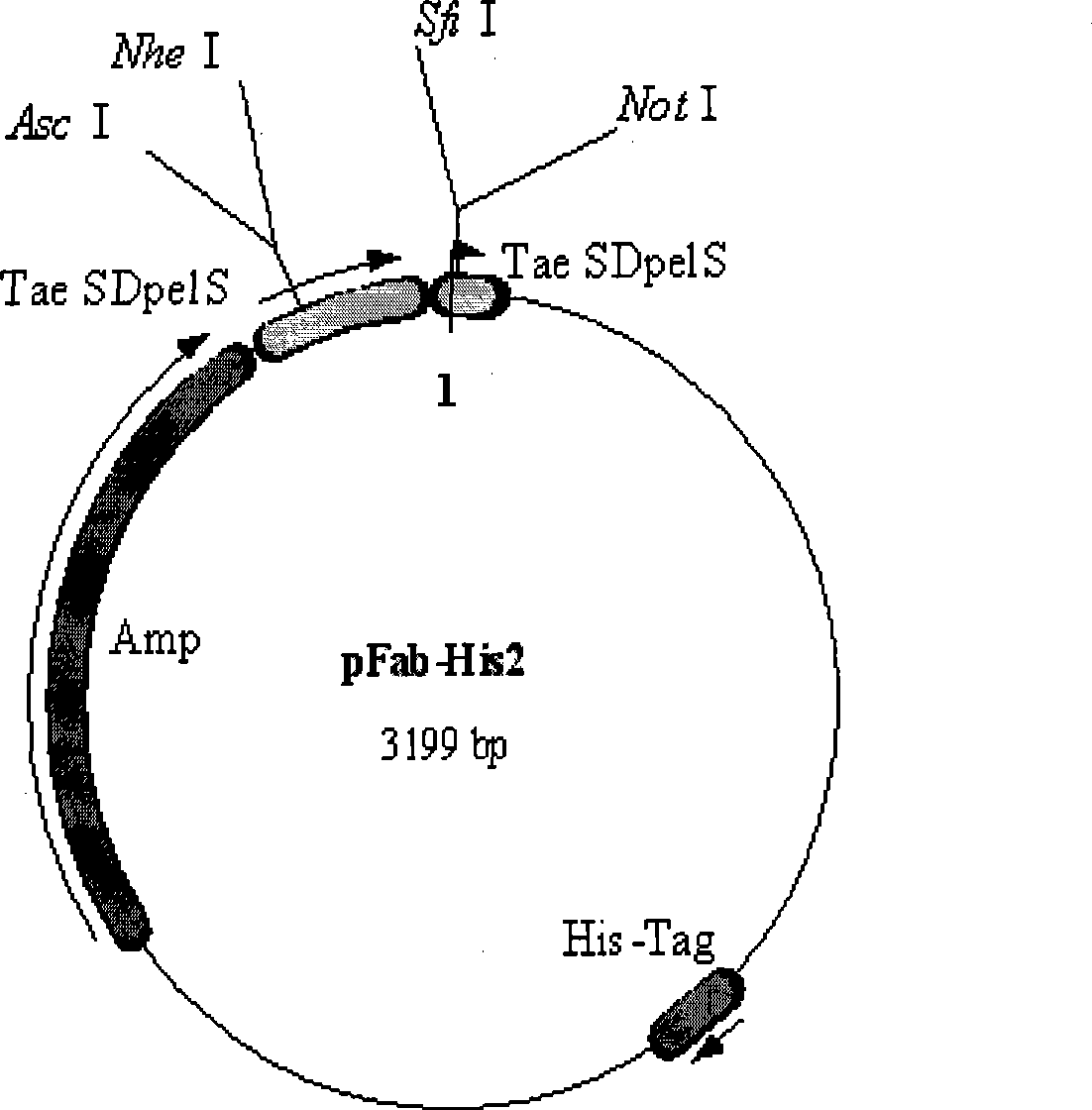
[0056] After the PCR products were purified, the κ chain and λ chain products were double-digested with Asc I and Nhe I, respectively. The digested κ chain and λ chain products were connected to the human immunoglobulin Fab expression vector pFab-His2 ( figure 1 , figure 2 ), and then electroporated into JM109 competent cel...
Embodiment 2
[0062] Example 2: Antibody Library Screening
[0063] (1) ELISA identification:
[0064] Transform 100ul JM109 competent cells with 1ul plasmid from the constructed antibody library, spread evenly on LB plates containing 100ug / ml ampicillin, and culture at 37°C for 12-16h, until monoclonal colonies grow. Pick out the monoclonal colony and the negative control (transformation of JM109 competent cells with the pFab1-His2 plasmid without heavy and light chain inserted) respectively from the LBA plate, and inoculate them in 2ml of SBA (30 g of tryptone, 20 g of yeastextract, 10 g of MOPS per liter, 100ug / ml Ampicillin, pH7.0) culture medium, the backup plate was cultured at 37°C overnight, and stored at 4°C. Bacteria cultured to OD 600 =0.5~0.8, add IPTG with a final concentration of 0.1mM and induce overnight at 30°C. The next morning, centrifuge at 8000rpm×15min, discard the supernatant, resuspend in 250ulPBS (containing PMSF with a final concentration of 1mM), and centrifuge...
Embodiment 3
[0083] Example 3 hTFFab 148 Expression, purification and identification of
[0084] (1) hTFFab 148 expression and purification of
[0085] hTFFab 148 After the cloning plasmid is transformed into JM109 competent cells, spread it evenly on the LBA plate. After culturing overnight, pick a single clone colony and inoculate it in 10ml of SBA culture medium with shaking at 37°C×250rpm until OD 600 About 1.0, dilute to 1000ml SBA culture medium at 37℃×250rpm according to the ratio of 1:100 and continue to shake to OD 600 About 1.0, inoculated in 18L SBA medium in a 30L fermenter, 37℃×350rpm, pH7.0, dissolved oxygen 60%, continue to culture to OD 600 Add IPTG to a final concentration of 0.5mM at about 1.0 and induce expression at 30°C for 8h ( Figure 8 ), 4000rpm×30min, centrifuge at 4°C, collect the bacteria, and freeze them at -80°C.
[0086] According to the wet weight of bacteria, add 5ml NPI-10 (containing PMSF with a final concentration of 1mM) per gram of bacteria to re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com