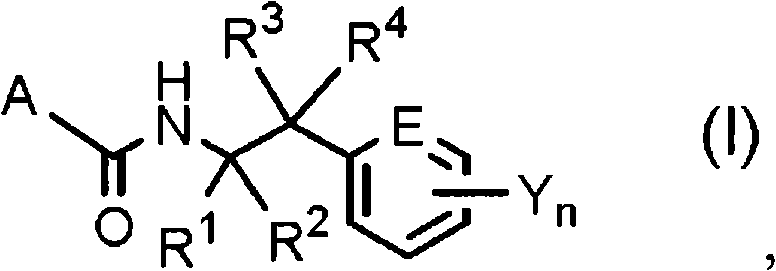
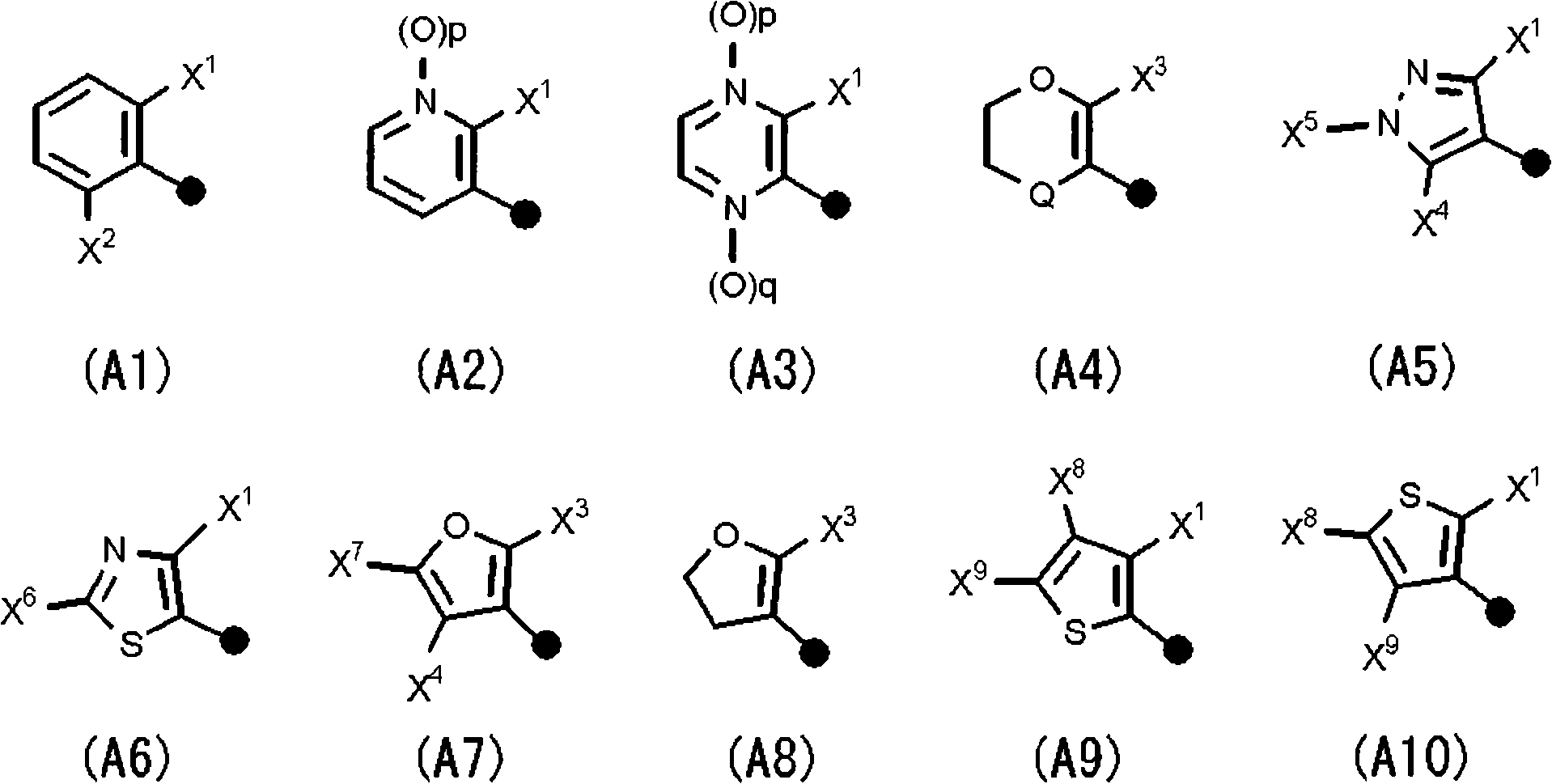
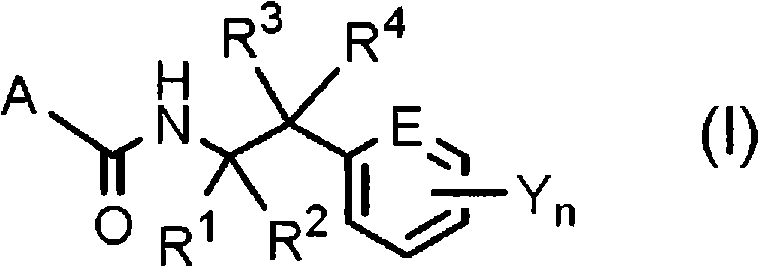
N-2-(hetero)arylethylcarboxamide derivative, and pest-controlling agent comprising the same
一种芳基乙基甲酰胺、衍生物的技术,应用在杀生物剂、用于生物控制的化学品、杀生剂等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0627] Preparation method of intermediate product 1 (when R 1 And R 2 When it is a hydrogen atom)
[0628]
[0629] Where R 3 , R 4 , E, Y and n are as defined above.
[0630] The above reaction can be carried out according to the method described in known publications (for example, Tetrahedron, 2002, 58(11), page 2211, etc.) or a method similar thereto. That is, the 2-(hetero)arylethylamine derivative represented by formula (III-1) can be produced as follows: in the presence of a base in an inert solvent, the acetonitrile derivative represented by formula (IV) and the corresponding The alkylating agent reacts to obtain the (hetero)arylacetonitrile derivative represented by the formula (V), and then, with or without separating the (hetero)arylacetonitrile derivative (V), the catalyst (for example, In the presence of Raney-nickel, etc., the hydrogenation reaction is carried out in an inert solvent, or the reduction reaction is carried out using a reducing agent (for example, lith...
Embodiment 1
[0782] Example 1. Preparation of N-[2-(2,4-Dichloro-phenyl)-1-methylethyl]-2-trifluoromethylbenzamide (Compound No. 1-32)
[0783] 1-1)
[0784]A mixture of 2,4-dichlorobenzaldehyde (1.45 g, 8.3 mmol), ammonium acetate (0.64 g, 8.3 mmol) and nitroethane (6 g, 80 mmol) was heated to reflux for 6 hours. The mixture was cooled to room temperature and ethyl acetate was added. The mixture was washed with water and saturated brine, then the mixture was dried with anhydrous magnesium sulfate, and then the mixture was concentrated. The concentrated mixture was recrystallized from ethanol to obtain 2,4-dichloro-1-(2-nitro-1-propenyl)benzene (yellow needle crystals, yield: 1.0 g, 52%).
[0785] 1-2)
[0786] At room temperature, a solution of 2,4-dichloro-1-(2-nitro-1-propenyl)benzene (1.0 g, 4.3 mmol) in tetrahydrofuran (5 ml) was added dropwise to the solution containing lithium aluminum hydride ( 0.33 g, 8.9 mmol) in tetrahydrofuran (20 ml) suspension. After that, reflux was started slow...
Embodiment 2
[0791] Example 2. N-[2-(2,4-Dichlorophenyl)-1-methylethyl]-2-trifluoromethylpyrazine-3-carboxamide (Compound No. 3-43) preparation
[0792] Add 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (0.3 g, 1.6 mmol) to the 2-trifluoromethylpyrazine-3-carboxylic acid ( 0.25 g, 1.3 mmol), [2-(2,4-dichlorophenyl)-1-methylethyl]amine (0.27 g, 1.3 mmol) and 4-dimethylaminopyridine (0.19 g , 1.6 mmol) in chloroform (10 ml) and the mixture was stirred at room temperature for 12 hours. After adding water and chloroform, the mixture was separated into layers, and the organic layer was washed successively with water and saturated brine and dried over anhydrous sodium sulfate. The mixture was concentrated under reduced pressure and the residue was purified by silica gel column chromatography (hexane:ethyl acetate=2:1) to obtain the desired compound (yield: 0.31 g, 63%).
[0793] Properties: The melting point is 163 to 164°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com