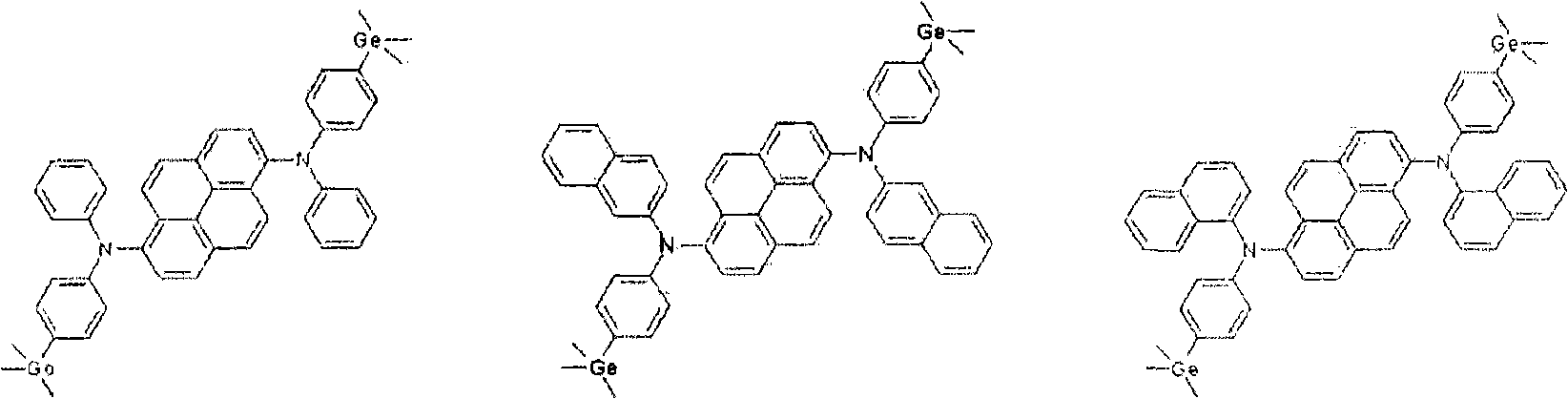
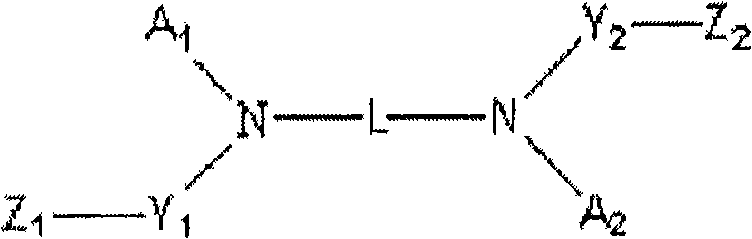New diamine derivatives, preparation method thereof and organic electronic device using the same
A technology of diamine derivatives and compounds, applied in the field of new diamine derivatives, their preparation and organic electronic devices using the diamine derivatives, can solve the problems of organic material layer materials for organic light-emitting devices that have not been fully realized, To achieve the effect of excellent life and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0171] Hereinafter, preferred embodiments of the present invention will be described for further understanding of the present invention. However, the following examples are presented for illustrative purposes only, and thus do not limit the scope of the present invention.
[0172] Compounds of general formula 1 according to the invention can be prepared in multistep chemical reactions. The preparation of this compound is described in the Examples below. It is clearly shown in the examples that a certain intermediate compound is prepared first, and then the compound of general formula 1 is prepared using the intermediate compound. Representative intermediate compounds are listed as compounds A to M below. In these compounds, "Br" may be substituted by any other active atom or functional group.
[0173]
[0174] [Compound A] [Compound B] [Compound C]
[0175]
[0176] [Compound D] [Compound E] [Compound F]
[0177]
[0178] [Compound G] [Compound H] [Compo...
preparation Embodiment 1
[0181] Preparation Example 1 : Preparation of Compound A
[0182] Dibromobenzene (20 g, 84.78 mmol) was dissolved in anhydrous tetrahydrofuran (THF, 200 mL) under nitrogen atmosphere at room temperature. The solution was cooled to -78°C. n-BuLi (34 mL, 2.5M in pentane) was slowly added to the solution at -78°C, and the temperature of the mixture was slowly raised to 0°C over about 1 hour. To the mixture was added trimethylgermanium bromide (18 ml, 101.74 mmol), and the temperature of the mixture was raised to room temperature over 1 hour. After confirming that the reaction was complete, the mixture was extracted from ethyl acetate, dried over magnesium sulfate, and distilled under reduced pressure to obtain Compound A (20 g, 90%). MS(M+)273
preparation Embodiment 2
[0183] Preparation Example 2 : Preparation of Compound B
[0184] Under nitrogen atmosphere, compound A (18g, 65.45mmol), aniline (6.6ml, 72mmol), pd(dba) 2 (0.125g, 0.13mmol), P(t-Bu) 3 (0.04 g, 0.2 mmol) and sodium tert-butoxide (1.80 g, 18.7 mmol) were added to toluene (200 mL), and the mixture was refluxed for about 3 hours. After the reaction was complete, the mixture was cooled to room temperature, and the reaction mixture was added to THF and H 2 in a mixed solution of O. The organic layer was separated, dried over magnesium sulfate and then concentrated. The residue was separated by column chromatography to obtain compound B (16 g, 85%). MS[M]=286
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com