1-vinyl-3-sulfobutyl imidazole bisulfate and preparation method thereof
The technology of sulfobutylimidazole salt and sulfobutylimidazole is applied in the field of acidic ionic liquid, which can solve the problem of easy loss of ionic liquid, and achieve the effects of small loss of active components, high stability and high mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of acidic ionic liquid 1-vinyl-3-sulfobutylimidazolium bisulfate:
[0027] ①Get 0.50mol of 1-vinylimidazole and 0.55mol of 1,4-butane sultone, place in a 250ml three-necked flask, and react at room temperature for 96 hours to obtain a white zwitterionic solid. The zwitterionic solid was washed successively with toluene and anhydrous ether, and then vacuum-dried at 40°C to constant weight to obtain the zwitterionic 1-vinyl-3-sulfobutylimidazolium salt.
[0028] ②Put 0.50mol of 1-vinyl-3-sulfobutylimidazolium salt into a four-neck flask, and slowly add 0.48mol of concentrated sulfuric acid (95%-98% by mass) dropwise while keeping the temperature below 10°C , the same below), and then reacted at room temperature for 6 hours, washed the reaction solution with toluene and anhydrous ether in sequence, and dried it in vacuum at 40°C to constant weight to obtain the target product acidic ionic liquid 1-vinyl-3- Sulfobutyl imidazolium bisulfate.
Embodiment 2
[0030] Synthesis of acidic ionic liquid 1-vinyl-3-sulfobutylimidazolium bisulfate:
[0031] ① Take 0.50mol of 1-vinylimidazole and 0.50mol of 1,4-butane sultone, put them in a 250ml three-necked flask, and react at 50°C for 12 hours to obtain a white zwitterionic solid. The zwitterionic solid was washed successively with toluene and anhydrous ether, and then vacuum-dried at 40° C. to constant weight to obtain the zwitterionic 1-vinyl-3-sulfobutylimidazolium salt.
[0032] ②Take 0.50mol of 1-vinyl-3-sulfobutylimidazolium salt into a four-necked flask, and slowly add 0.52mol of concentrated sulfuric acid dropwise while keeping the temperature below 10°C, and then react at 40°C for 3 After several hours, the reaction solution was washed with toluene and anhydrous ether in sequence, and then vacuum-dried at 40° C. to constant weight to obtain the target acidic ionic liquid 1-vinyl-3-sulfobutylimidazolium hydrogensulfate.
Embodiment 3
[0034] Synthesis of acidic ionic liquid 1-vinyl-3-sulfobutylimidazolium bisulfate:
[0035] ①Take 0.50mol of 1-vinylimidazole and 0.52mol of 1,4-butane sultone, place them in a 250ml three-necked flask, and react at 30°C for 48 hours to obtain a white zwitterionic solid. The zwitterionic solid was washed successively with toluene and anhydrous ether, and then vacuum-dried at 40° C. to constant weight to obtain the zwitterionic 1-vinyl-3-sulfobutylimidazolium salt.
[0036] ②Put 0.50mol of 1-vinyl-3-sulfobutylimidazolium salt into a four-necked flask, and slowly add 0.50mol of concentrated sulfuric acid dropwise while keeping the temperature below 10°C, and then react at 30°C for 4 After several hours, the reaction solution was washed with toluene and anhydrous ether in sequence, and then vacuum-dried at 40° C. to constant weight to obtain the target acidic ionic liquid 1-vinyl-3-sulfobutylimidazolium hydrogensulfate. The molecular weight is 328.
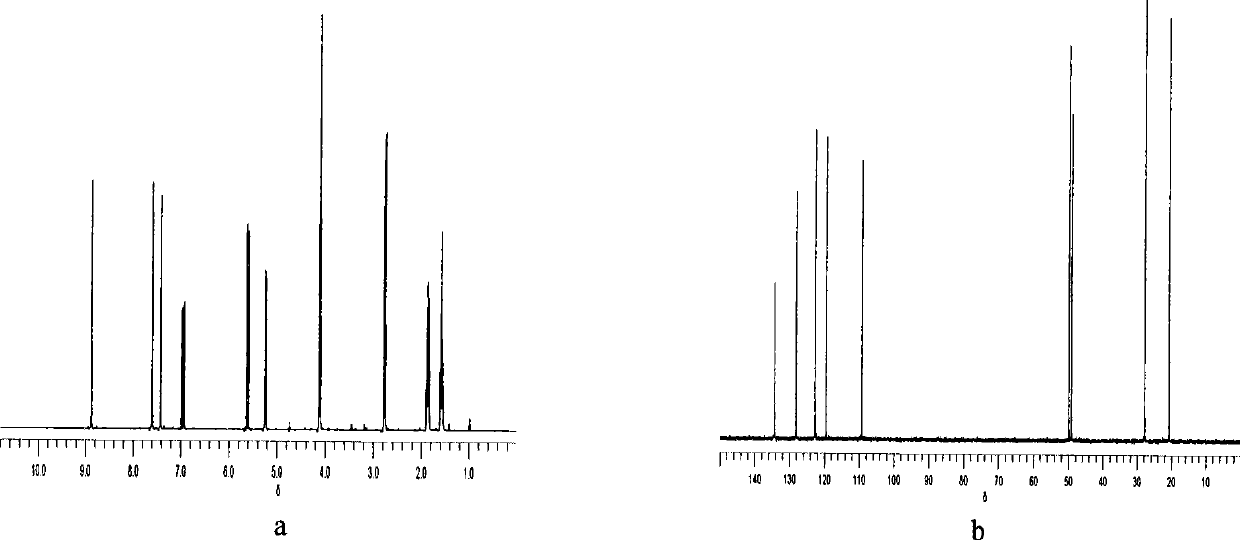
[0037] The characterization...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com