Rifamycin analogs and uses thereof
一种动物、卤素的技术,应用在洗涤剂组合物、有机化学、化学仪器和方法等方向,能够解决菌株数量增长、无法有效治疗等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Example 1: General Coupling Method
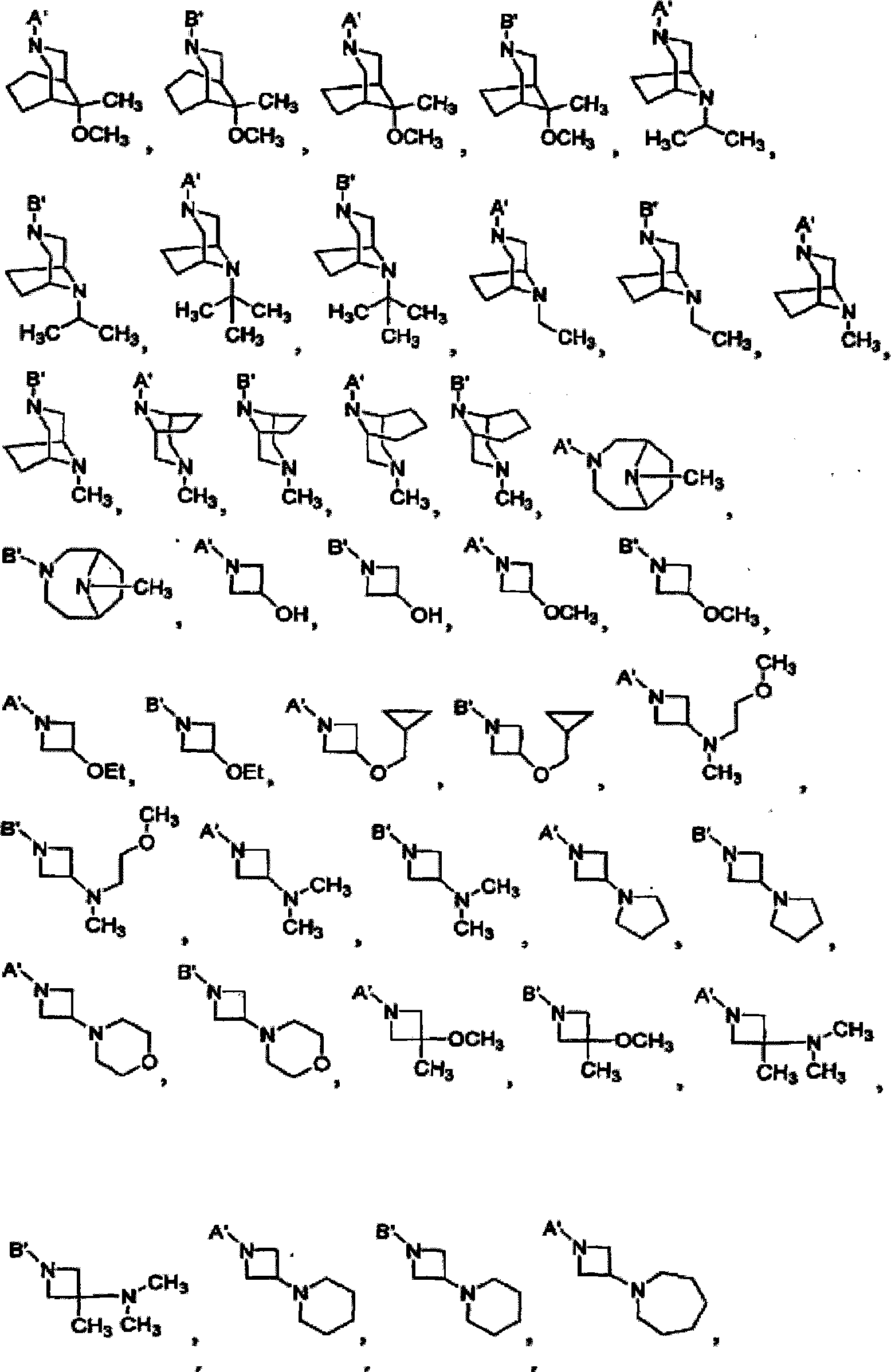
[0171] Synthetic 5'-substituted benzoxazine rifamycins, 5'-substituted benzothiazine rifamycins, and 5'-substituted benzodiazines rifamycins can all follow the same procedure listed in Scheme 1 The general route is carried out using the general method disclosed in US Patent No. 4,965,261 to add the amino group to the 5'-position. In this scheme, rifamycin azaquinone of formula II is dissolved in a suitable solvent, such as DMSO, and then reacted with amine or formula III in the presence of manganese dioxide at room temperature for several hours to form azaquinone of formula IV . If desired, the azabenzoquinone of formula IV can be further reacted with a deprotecting reagent to remove the P' and / or any P" protecting groups introduced at the 25-position, X, 21 and 23 positions in the amine of formula III In some embodiments, a group that is beneficial to pharmacokinetic properties can be further introduced at the 25-position, for exa...
Embodiment 2
[0180] Example 2: General procedure for deacetylation
[0181] A compound of formula VI (-100 mmol) was dissolved in methanol (5 mL) and treated with saturated sodium hydroxide in methanol (5 mL) at room temperature for 0.5 h-3 h. The reaction mixture was poured into saturated ammonium chloride solution, and extracted with chloroform. The organic phase was washed with water (2×), Na 2 SO 4 dry. After filtration the solvent is removed in vacuo to give the desired deacetylated product of formula X. If desired, the product can be purified by flash chromatography (silica gel) using an appropriate solvent system (eg 1-10% methanol in dichloromethane).
[0182]
Embodiment 3
[0183] Example 3: Synthesis of Compound 1 (3'-hydroxyl-5'-(3-hydroxyl-1-azetidinyl)benzoxazine rifamycin), (see Table 1 for structure)
[0184] The title compound was prepared using the general coupling procedure of Example 1 using compound 100 (1 mmol), 3-hydroxyoxazetidine, TBDMS ether (2.5 mmol) and manganese(IV) oxide (10 mmol). Removal of the silyl protecting group with tetrabutylammonium fluoride affords compound 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com