Use of alpha-hydroxy carbonyl compounds as reducing agents
A technology for compounds and organic compounds, applied in the direction of active ingredients of hydroxy compounds, active ingredients of nitro compounds, organic reduction, etc., can solve the problems of non-disclosure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
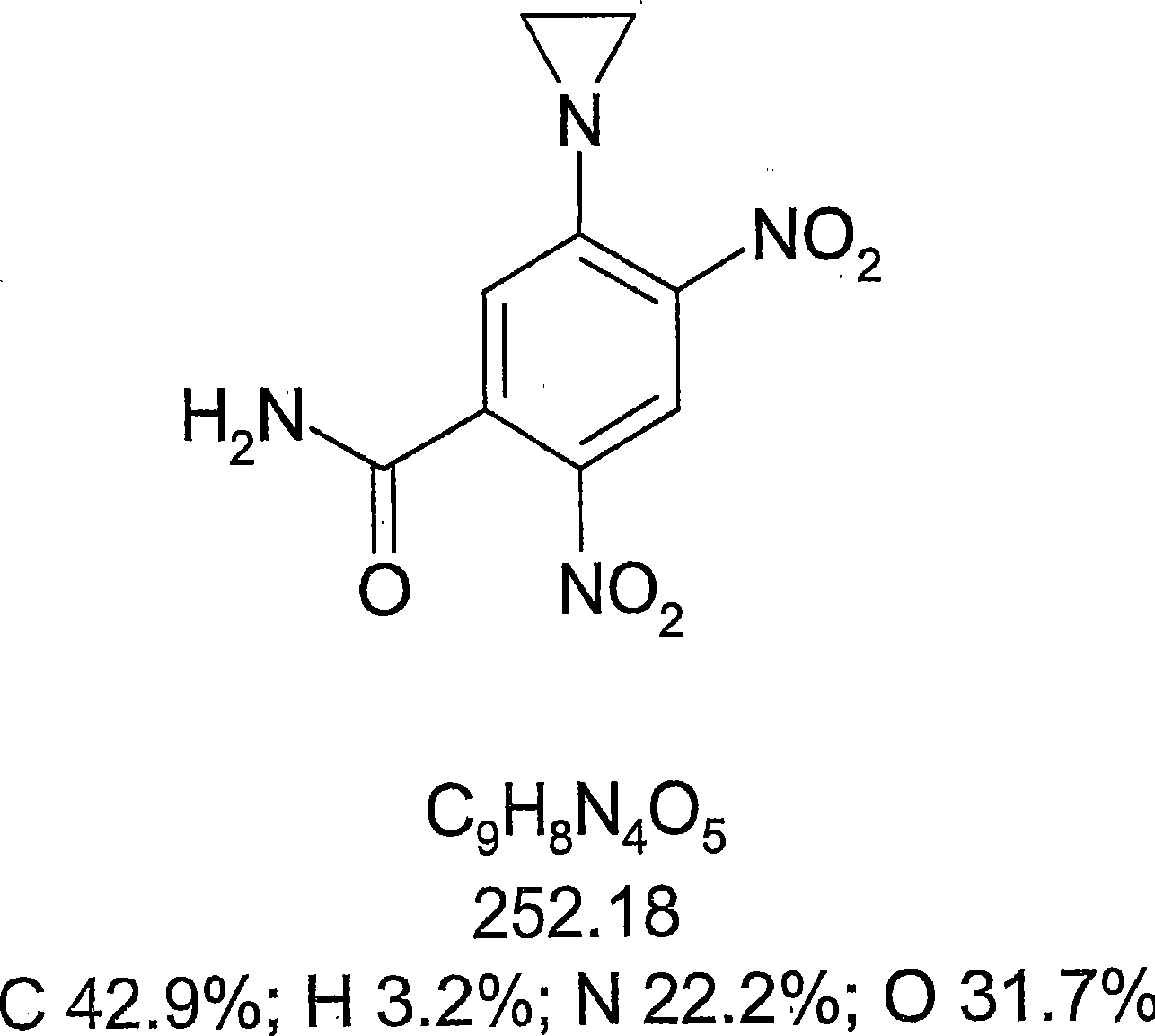
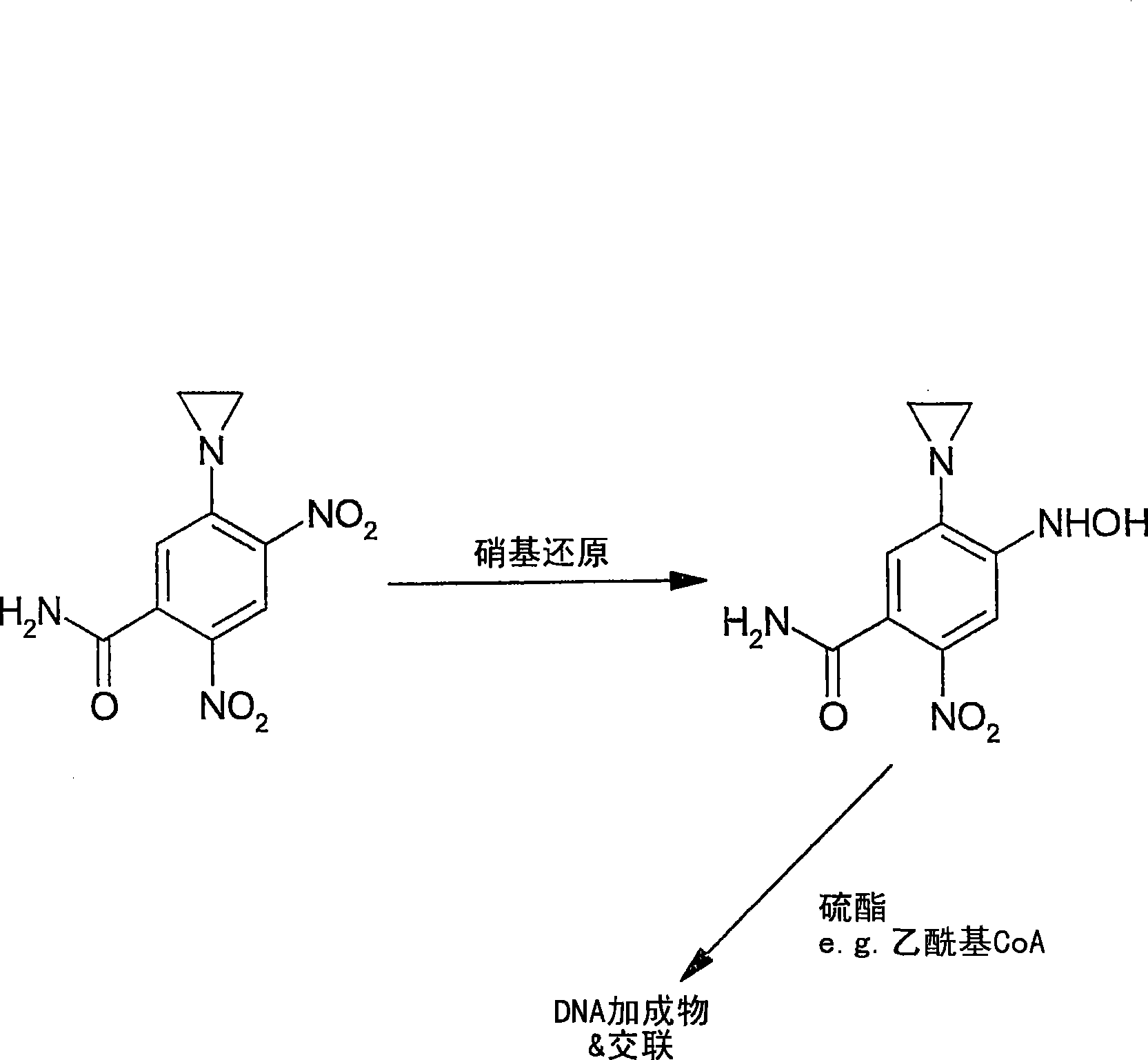
[0374] Example 1: Chemical Activation of Tretazicar
[0375] Reported chemistries for the manufacture of active 4-hydroxyamino derivatives from tretazicar use harsh reducing conditions in organic solvents to achieve yields below 30% (Knox et al., 1993; Knox et al., 1988). The inventors have found that dihydroxyacetone (DHA) is capable of reducing tretazicar to the desired hydroxylamine in aqueous solution under mildly basic conditions. At pH 9, the yield was >85%, and the only other product detected by tretazicar reduction was 5-(aziridin-1-yl)-2-hydroxyamino-4-nitrobenzamide.
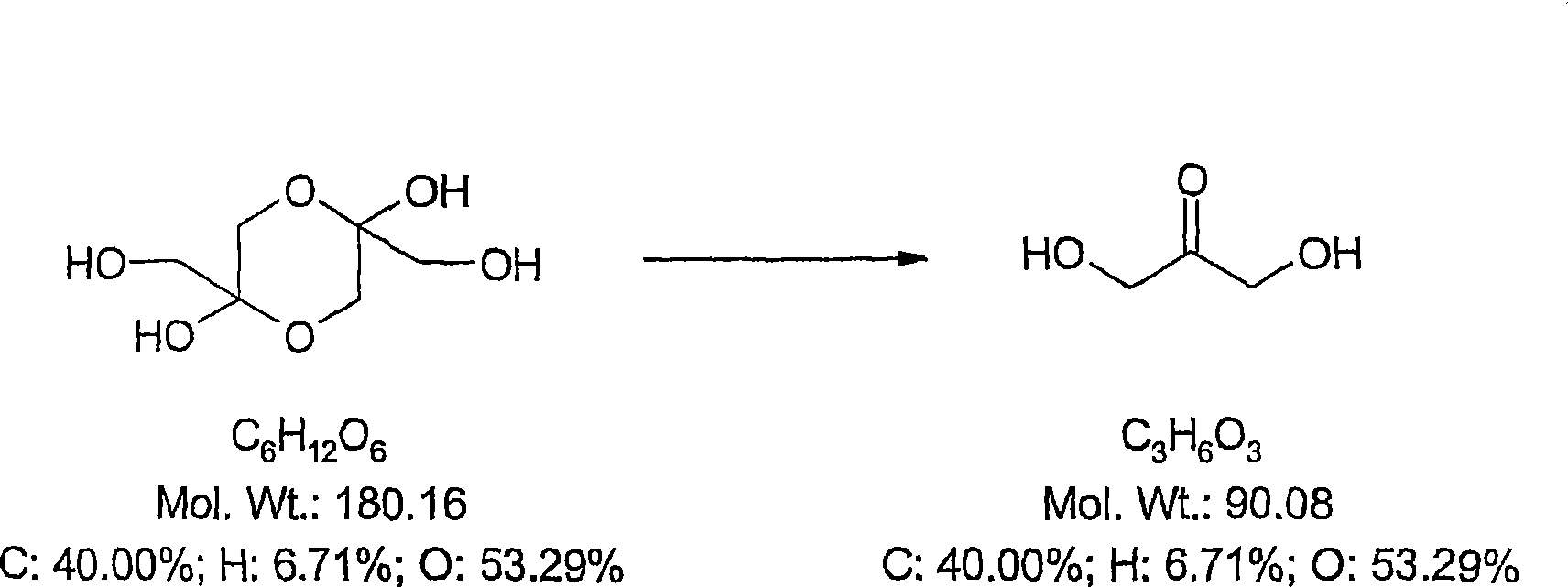
[0376] Dihydroxyacetone (DHA; 1,3-dihydroxy-2-propanone; CAS No: 62147-49-3, Beil.8, 266, Merck Index 13, 3166; image 3 ) is the active ingredient in sunscreen or self-tanning lotions and is FDA-approved. DHA is a colorless sugar that darkens the color of the skin by staining. It interacts with dead surface cells in the epidermis to produce a color shift. Generally within 5 to 7 days after applica...
Embodiment 2
[0387] Example 2: Activation of tretazicar in emulsion for topical administration
[0388] Two emulsions designated A and B were prepared. To use, mix them in equal amounts. Emulsion A consisted of E45 base (white soft paraffin BP 14.5% w / w, light liquid paraffin Ph Eur 12.6% w / w, hypoallergenic anhydrous lanolin (Medilan) 1.0% w / w, Crookes Healthcare Ltd, Nottingham, UK ) composition), each gram contains 10mg tretazicar, 10mg NaHCO 3 and 90mg Na 2 CO 3 . Emulsion B contained E45 and contained 100 mg DHA dimer per gram. Components A and B were mixed to obtain a pale yellow emulsion. Turns brown after a few hours and continues to deepen over 24 hours. A suspension of 200 μg of the emulsion in 1 mL of water yielded a solution of approximately pH 10 as indicated by pH paper under vigorous stirring. Preliminary experiments with emulsions containing 10% of the above amounts of buffer salts gave solutions of the same initial pH. However, after 4 hours, the solution prepared...
Embodiment 3
[0393] Embodiment 3: External use of tretazicar emulsion
[0394] The above-prepared emulsion was mixed, and about 0.1 g was applied to the warts (growing, slope height 1.5 mm) on the fingers of healthy human volunteers, and covered with a plaster. The initial temperature (warmth) at which the emulsion was applied was recorded. After approximately 4 hours, the plaster was removed and the wart was observed to have fallen off, leaving a ~1 mm deep pit. The surrounding tissue turns yellow. After a few days, it gradually turned white, and no regrowth of the wart was observed after 6 weeks. There were also no reports of significant side effects.
[0395] Each gram contains 10mg tretazicar, 10mg NaHCO 3 and 90mg Na 2 CO 3 Emulsion A (composed of E45 base (white soft paraffin BP 14.5% w / w, light liquid paraffin Ph Eur 12.6% w / w, hypoallergenic anhydrous lanolin (Medilan) 1.0% w / w, Crookes Healthcare Ltd, Nottingham , composed of UK) mixed with lotion B (containing E45, contain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com