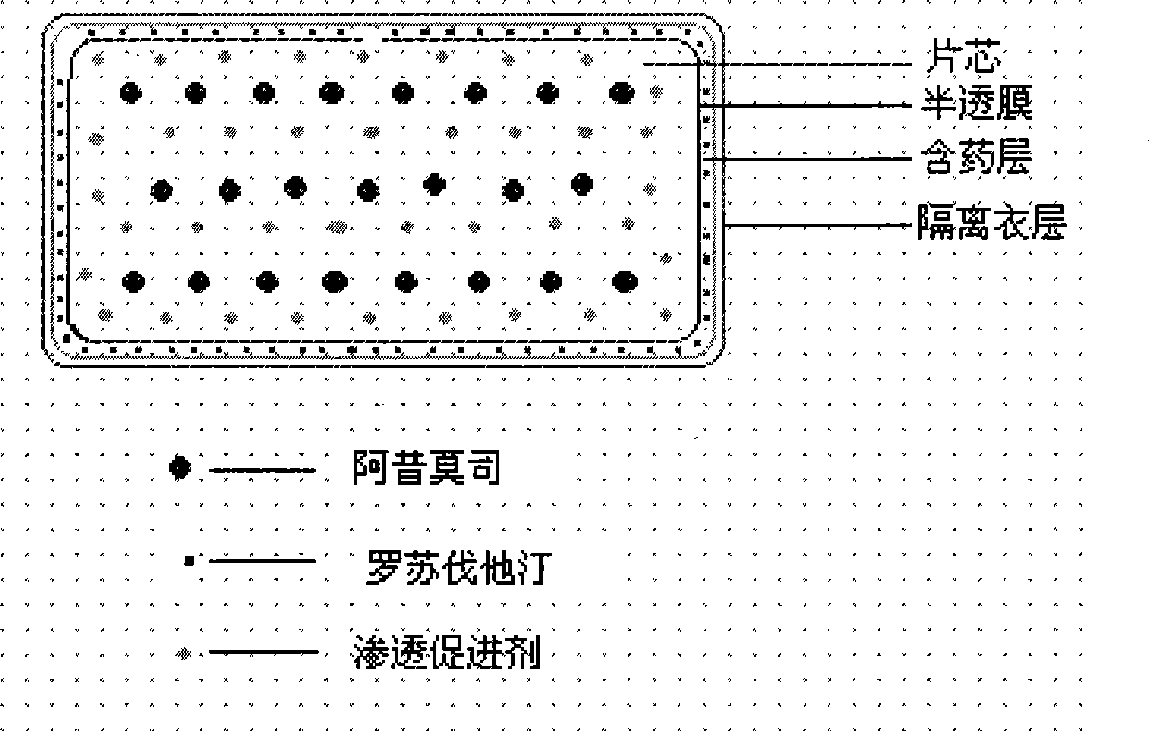
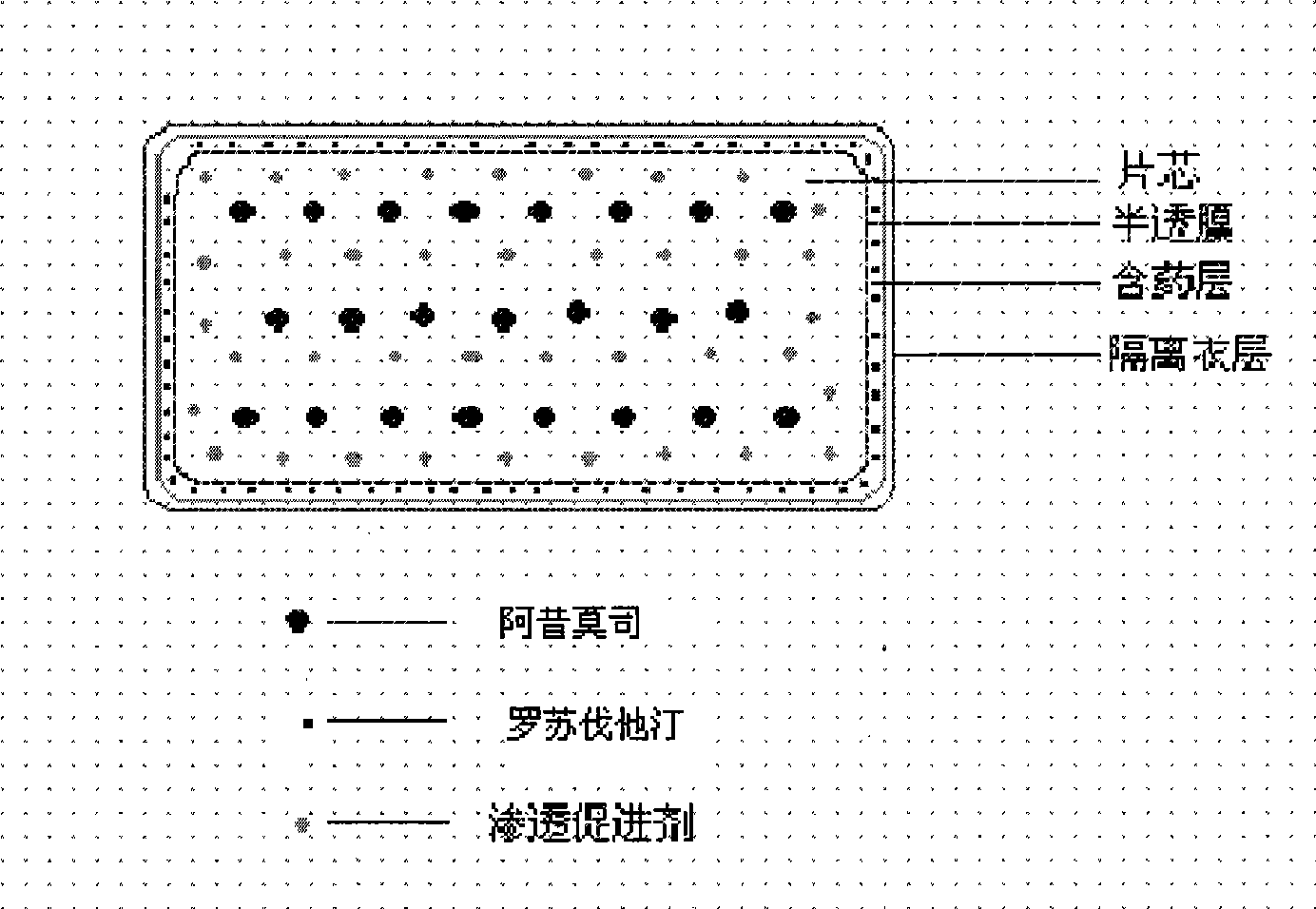
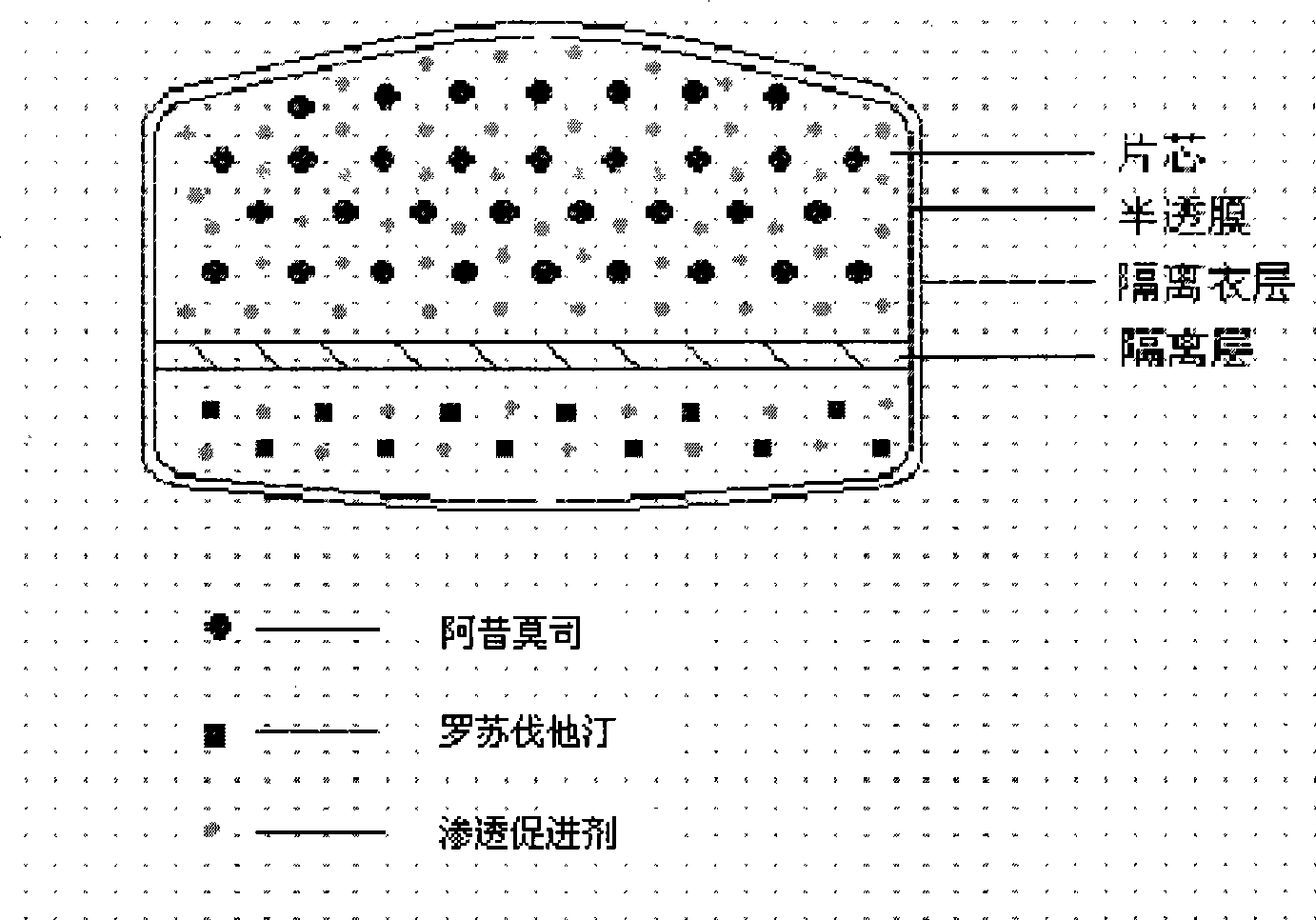
Osmotic pump controlled release preparation composition for treating hyperlipemia and preparation method thereof
A technology of osmotic pump controlled release and composition, which is applied in the field of medicine and can solve problems such as preparation of osmotic pump preparations that have not been mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Chip composition:
[0085] Acipimus 250g
[0086] NaCl 100g
[0087] PVPk30 5g
[0089] Coating film composition:
[0090] Cellulose acetate 12g
[0091] Macrogol 4000 3.5g
[0092] Diethyl phthalate 2g
[0093] Immediate release drug layer composition:
[0094] Rosuvastatin 4.5g
[0095] HPMC 6cp 2.5g
[0097] Isolation film coat layer:
[0098] Opadry II
[0099] Make 1000 tablets by the following preparation method: (1) Tablet core preparation Get sodium chloride and pulverize, cross 100 mesh sieves, mix with acipimox evenly, use 70% alcohol solution containing 8% PVPk30 as wetting agent, prepare Soft material, passed through a 20-mesh sieve to granulate, dried at 45°C for 2 hours, granulated, added with magnesium stearate, mixed evenly, compressed into tablets, and compressed into 1000 tablets by conventional tableting technology. (2) Tablet core coating: take cellulose acetate, add 280ml of acetone...
Embodiment 2
[0102] Chip composition:
[0103] Acipimus 250g
[0104] Fructose 50g
[0105] Lactose 50g
[0106] PVPk30 5g
[0108] Coating film composition:
[0109] Cellulose acetate 12g
[0110] Macrogol 4000 1.5g
[0111] Dibutyl sebacate 2g
[0112] Immediate release drug layer composition:
[0113] Rosuvastatin 8g
[0114] HPMC 6cp 5g
[0115] Sodium Lauryl Sulfate 2g
[0116] Titanium dioxide 1g
[0118] Make 1000 tablets by the following preparation method: (1) Tablet core preparation Get sodium chloride and pulverize, cross 100 mesh sieves, mix with acipimox evenly, use 70% alcohol solution containing 8% PVPk30 as wetting agent, prepare Soft material, passed through a 20-mesh sieve to granulate, dried at 45°C for 2 hours, granulated, added with magnesium stearate, mixed evenly, compressed into tablets, and compressed into 1000 tablets by conventional tableting technology. (2) Tablet core coating: take cellulose...
Embodiment 3
[0121] Chip composition:
[0122] Acipimus 250g
[0123] NaCl 100g
[0124] PVPk30 5g
[0126] Coating film composition:
[0127] Ethylcellulose 12g
[0128] HPMC6cp 3g
[0129] Macrogol 4000 1g
[0130] Immediate release drug layer composition:
[0131] Rosuvastatin 12.5g
[0132] HPMC 6cp 7g
[0134] Isolation film coat layer:
[0135] Opadry II
[0136] Make 1000 tablets by the following preparation method: (1) tablet core preparation Get sodium chloride and pulverize, cross 100 mesh sieves, mix with acipimox evenly, take the 50% ethanol solution containing 5% HPMC6cp as wetting agent, prepare Soft material, passed through a 20-mesh sieve to granulate, dried at 5°C for 2 hours, granulated, added with magnesium stearate, mixed evenly, compressed into tablets, and compressed into 1000 tablets by conventional tableting technology. (2) Core coating: take ethyl cellulose, add 320ml of ethanol, stir to dissolve; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com