Topical mecamylamine formulations for ocular administration and uses thereof
A mecamylamine, topical application technology, applied in the direction of medical preparations of non-active ingredients, drug delivery, amine active ingredients, etc., can solve the problems of not being commercially available, difficult to model nicotine, and not surprising
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0276] Embodiment 1: mecamylamine parenteral preparation
[0277]
[0278] A parenteral formulation of mecamylamine hydrochloride was prepared by substantially dissolving 1 g of mecamylamine hydrochloride USP (white powder) and 33.33 mL of 0.9% sterile NaCl in a volumetric flask. The mixture was manually stirred at room temperature until the mecamylamine powder was completely dissolved to give a clear solution. The pH of the solution was adjusted to 7.4 using NaOH and HCl.
Embodiment 2
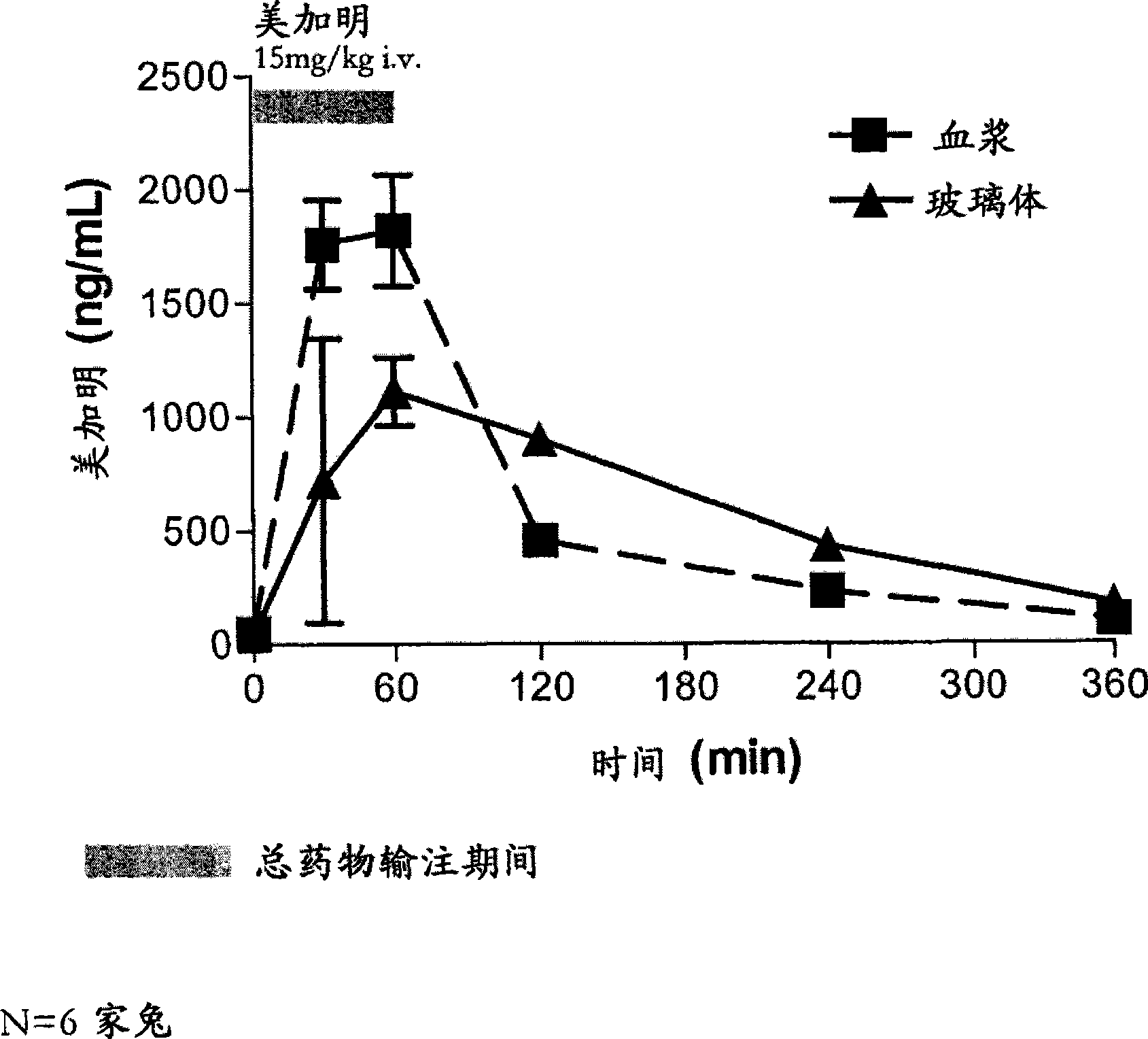
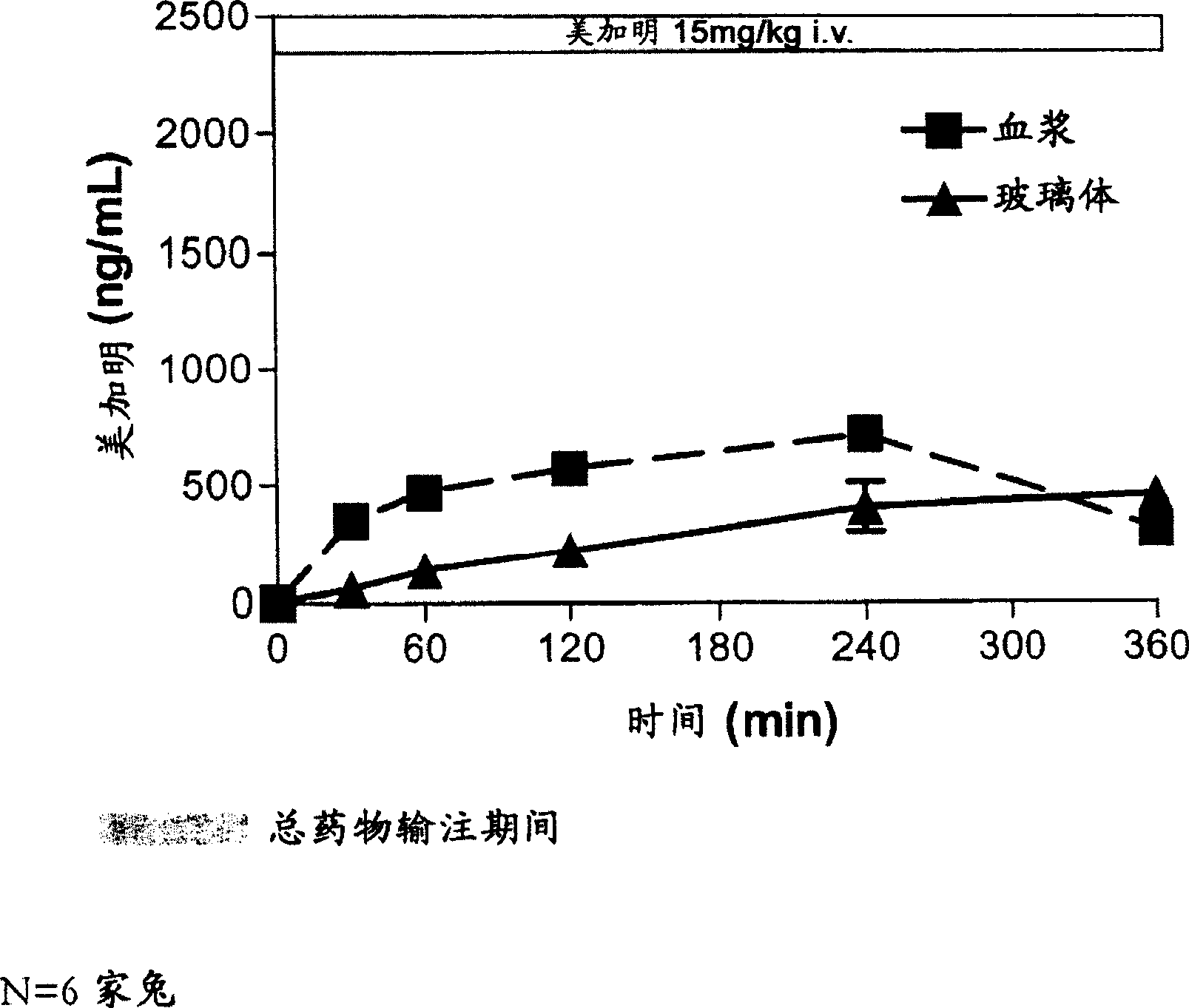
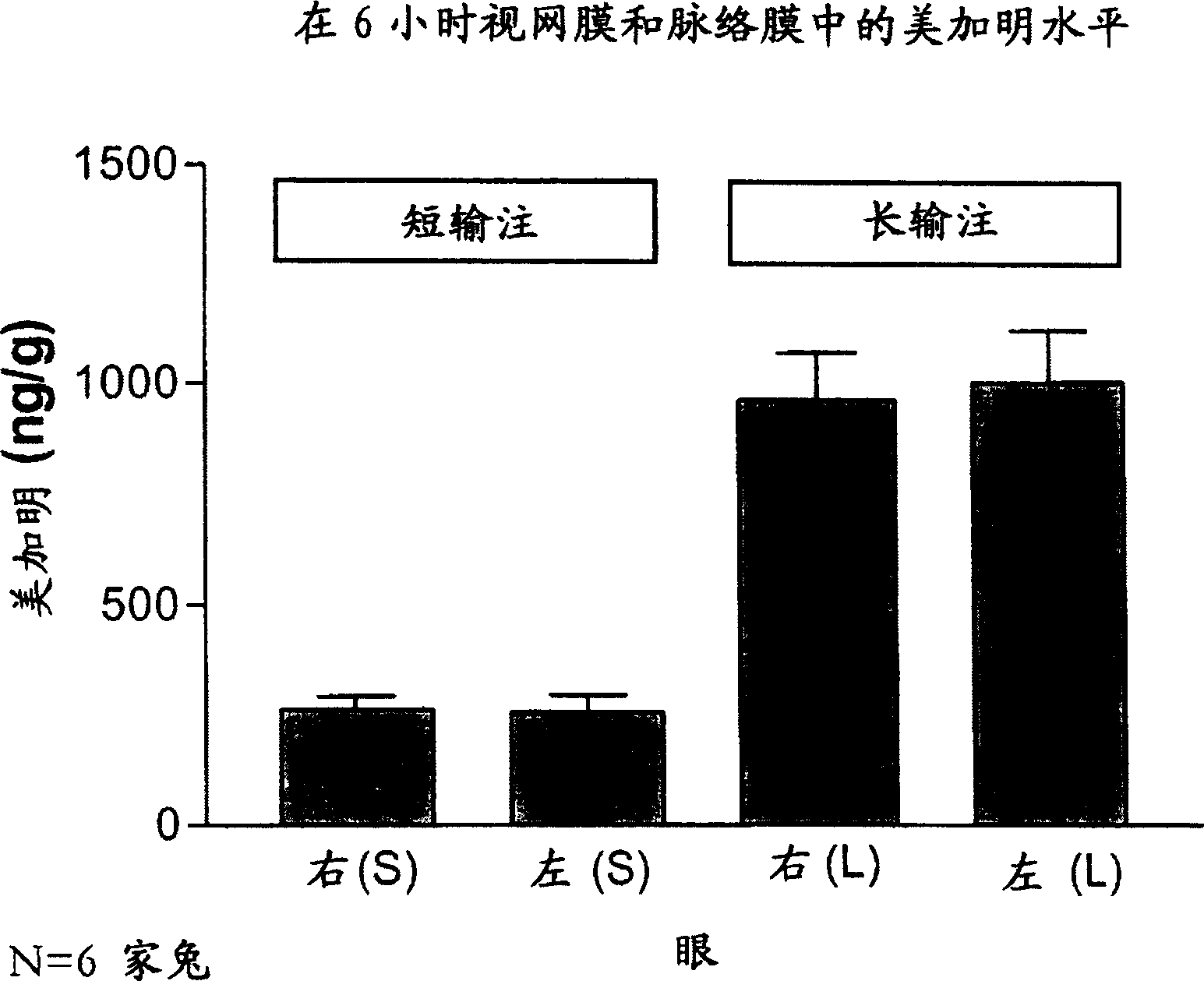
[0279] Example 2: Ocular Bioavailability After Intravenous Administration
[0280] This study was designed to model the ocular bioavailability of mecamylamine when administered systemically. The rabbit eye is the preferred model for in vivo modeling of ophthalmic drugs, however, rabbits are not the subject of choice for modeling oral bioavailability. However, systemic administration does mimic orally administered mecamylamine to a reasonable approximation due to mecamylamine's rapid absorption and high oral bioavailability. Therefore, intravenous injection was used to model the ocular bioavailability of systemically administered mecamylamine in order to determine the disposition of mecamylamine from blood to plasma, vitreous, and posterior ocular tissues (retina / choroid).
[0281] The study included 2 groups (N = 6 each, 12 rabbits in total) of male NZW (New Zealand White) rabbits weighing approximately 2.5-3 kg and obtained from Kralek Farms (Turlock, CA). A solution of mec...
Embodiment 3
[0285] Example 3: Preparation of Topical Ophthalmic Solution Formulations
[0286]
[0287] Dissolve mecamylamine hydrochloride USP in 100 mL of DI water. Then 0.9 g weight of sodium chloride was added while stirring to make an isotonic solution (0.9% NaCl w / v). The solution was then filtered through a 0.2 micron membrane filter and packaged under sterile conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com