Solid pharmaceutical composition comprising irbesartan
A technology for solid pharmaceutical preparations and mixtures, which is applied in the field of irbesartan hydrochloride and can solve problems such as low solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0112] Tablets are prepared according to the following wet granulation method (A).
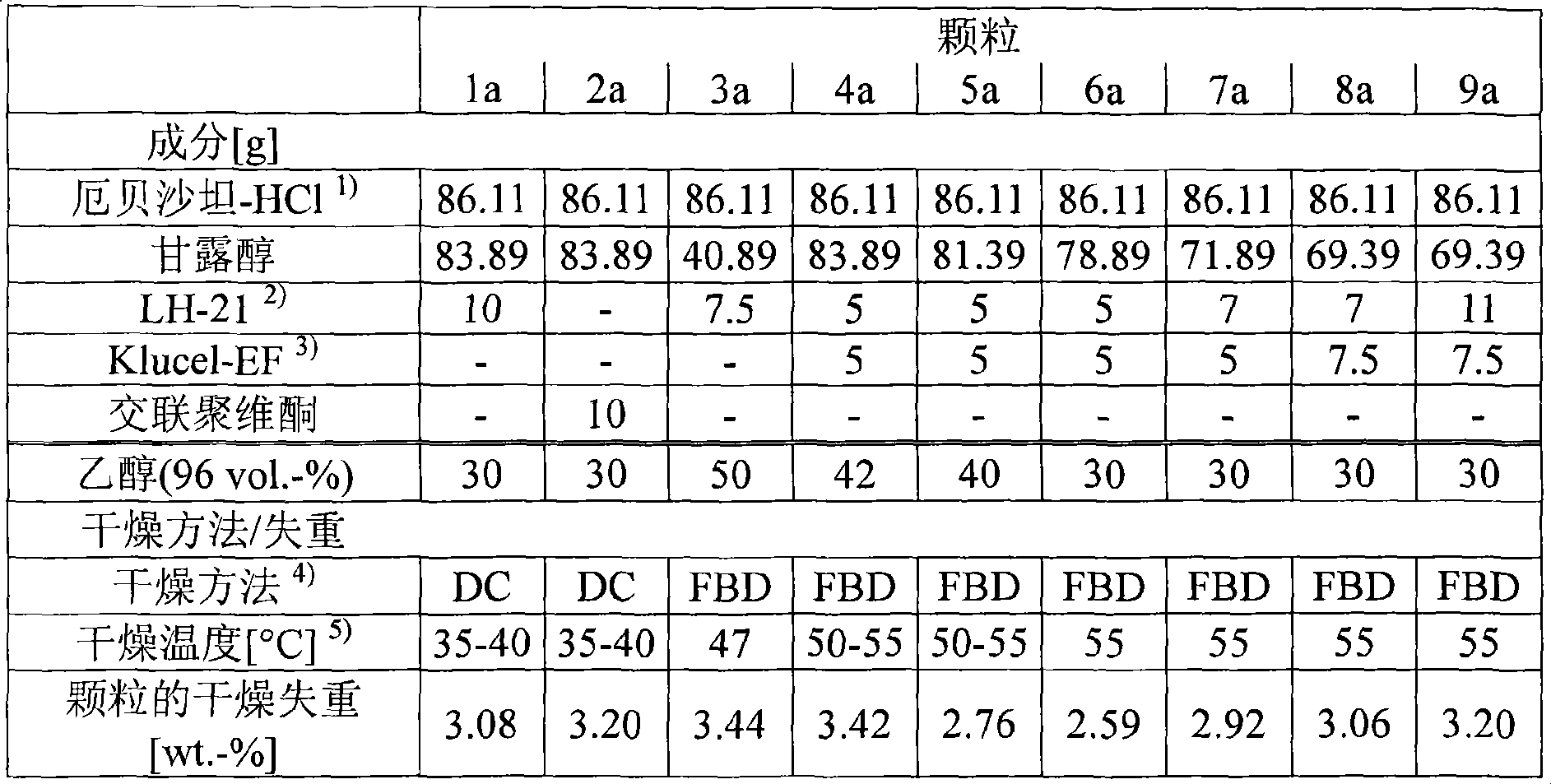
[0113] 1) Preparation of particles (1a)-(9a)
[0114] The solid components in the following Table 1 were respectively mixed in a high shear mixer, and ethanol (96 vol.-%) was sprayed on the mixture. The resulting granules are dried at a specific temperature in a drying chamber or in a fluidized bed dryer. The resulting granules were sieved and the loss on drying was determined as previously described. The properties and amounts of solid components and ethanol, drying equipment, drying temperature and drying loss of sieved particles are shown in Table 1.
[0115] Table 1
[0116]
[0117] 1) Is Irbesartan-HCl*1.5H 2 O(86.11g Irbesartan-HCl*1.5H 2 O equivalent to 75g free irbesartan)
[0118] 2) LH-21: commercially available low-substituted hydroxypropyl cellulose
[0119] 3) Klucel-EF: commercially available low-substituted hydroxypropyl cellulose
[0120] 4) DC: drying room
[...
Embodiment 10
[0137] Tablets were prepared according to direct compression method (b) as follows.
[0138] 86.11 g irbesartan-HCl, 40.89 g mannitol and 8 g croscarmellose sodium were mixed in a high shear mixer. Thereto were added 1.50 g of magnesium stearate, 1.5 g of anhydrous silicon dioxide and 12 g of talc, whereby a tableting mixture was obtained. The loss on drying was determined to be 3.1 wt.-% according to the method described above.
[0139] The tableting mixture is compressed into oval tablets with a theoretical weight of 150 mg. The hardness of the tablet core was 45-60N, and its disintegration time in pure water at 37°C was 20 seconds.
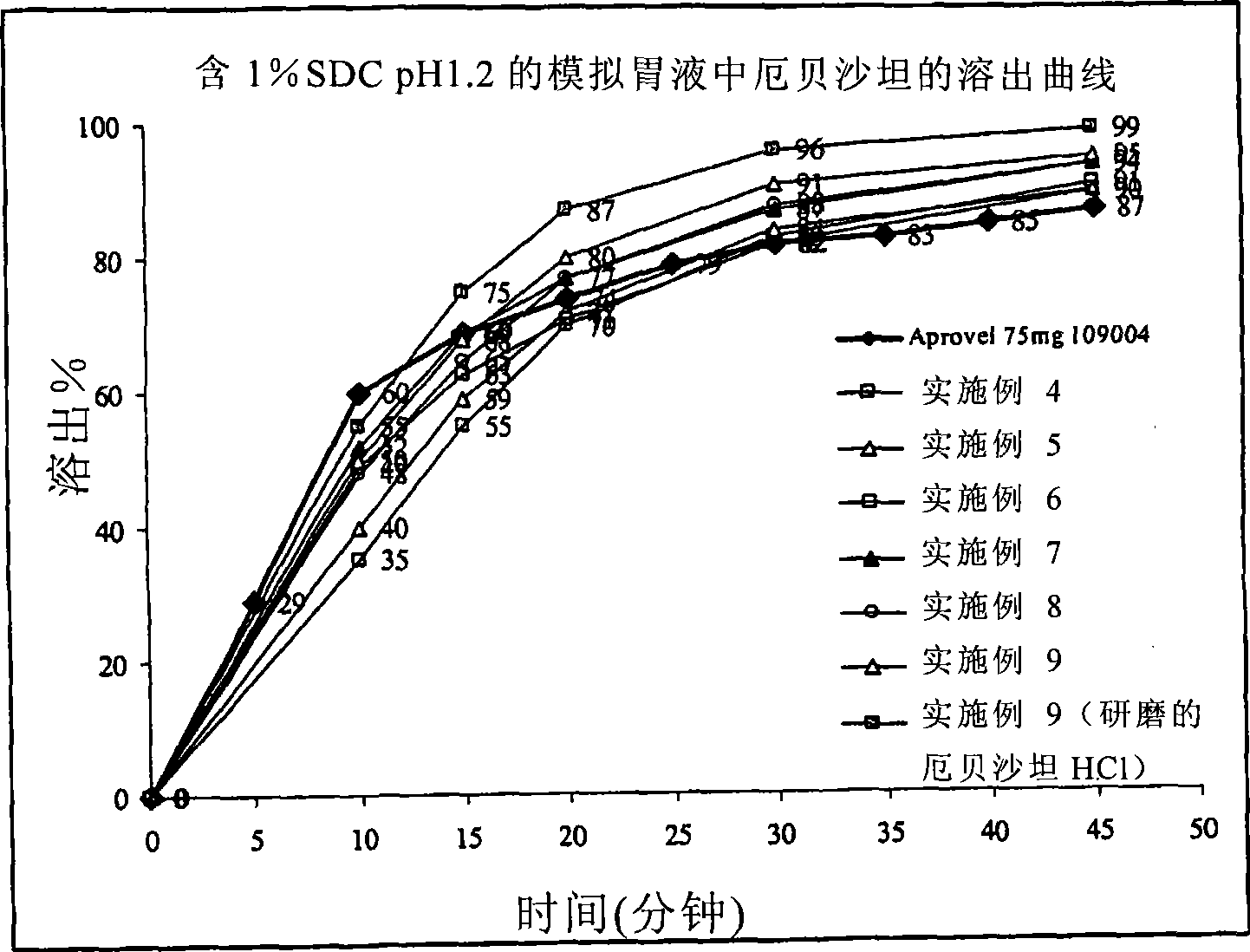
[0140] figure 1 Shown to contain 75 mg free irbesartan (equivalent to irbesartan-HCl*1.5H 2 (286.11mg) tablet core (4c)-(9c) of Examples 4-9 of the present invention and the comparison of the dissolution properties of the pharmaceutical dosage form (Aprovel75mg tablet) currently on the market. It can be seen that the pharmaceutical formula...
Embodiment 11-16
[0143] Tablets are prepared according to the following wet granulation method (A), adding hydrochlorothiazide, the steps are as follows: 1) Preparation of granules (11a)-(18a)
[0144] The solid components in the following Table 4 were respectively mixed in a high shear mixer, and ethanol (96 vol.-%) was sprayed on the mixture. The resulting granules are dried in a drying chamber or in a fluid bed drier. The nature and amount of solid components are shown in Table 4.
[0145] Table 4
[0146]
[0147] 1) Is Irbesartan-HCl*1.5H 2 O(86.11g Irbesartan-HCl*1.5H 2 O equivalent to 75g free irbesartan)
[0148] 2) LH-21: commercially available low-substituted hydroxypropyl cellulose
[0149] 3) q.s.: Appropriate amount
[0150] 2) Preparation of tableting mixture (11b)-(18b)
[0151] The ingredients shown in Table 5 below were respectively added to the granules (11a) to (18a) obtained above, followed by mixing in a high shear mixer to obtain a tableting mixture. The amo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com