Catalytic process for the oligomerization of olefinic monomers
A technology of olefin monomer and catalyst, applied in the field of olefin monomer oligomerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
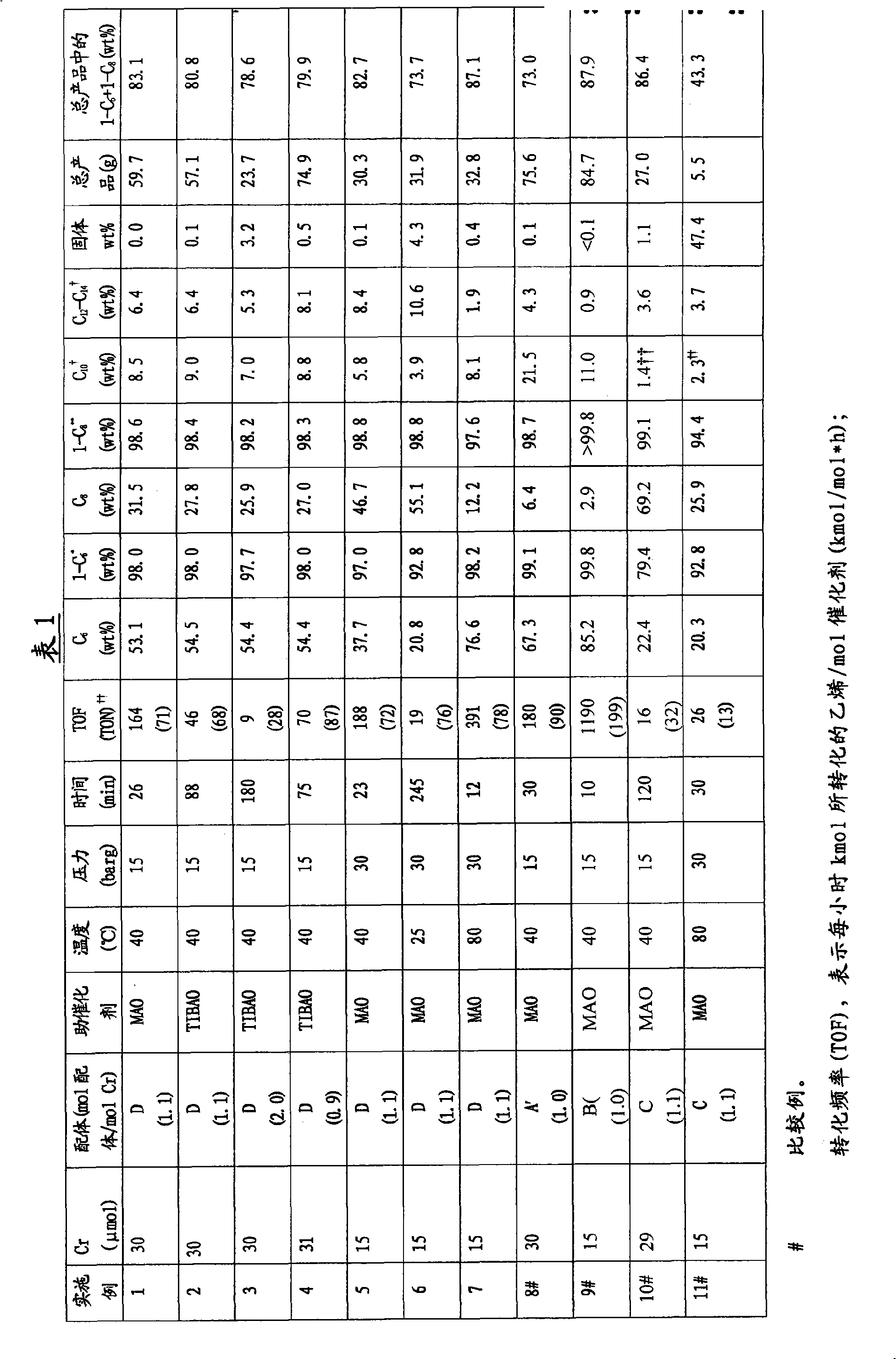
Embodiment 1-11
[0167] Catalyst system preparation for simultaneous trimerization and tetramerization in a batch autoclave
[0168] CrCl in a Braun MB 200-G drying oven 3 The 1:1 complexes with Ligand A or B (ie composition A' or B' indicated in Table 1) were placed in glass vials. The catalyst precursor composition was converted to a catalyst solution by adding a 3 or 1.5 mmol solution of MAO in toluene (approximately 1.6 g or 0.8 g MAO solution), followed by typically 4 g of anhydrous toluene. Finally the bottle is sealed with a septum cap.
[0169] These catalyst solutions or part of these solutions are used for the simultaneous trimerization and tetramerization of ethylene.
[0170] Alternatively, tris(acetylacetonate)chromium (typically 30 μmol) and the amount of ligand component C or D as shown in Table 1 were placed in a glass bottle, anhydrous toluene (typically 4 g) was added thereto, to obtain a catalyst precursor solution. Finally the bottle is sealed with a septum cap.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com