Method for enhance transport capability of gene mediated by non-viral vector
A non-viral and genetic technology, applied in biochemical equipment and methods, using vectors to introduce foreign genetic material, microorganisms, etc., can solve problems such as safety hazards and transportation capacity constraints, and achieve high safety and gene transportation capabilities. Improvement, low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
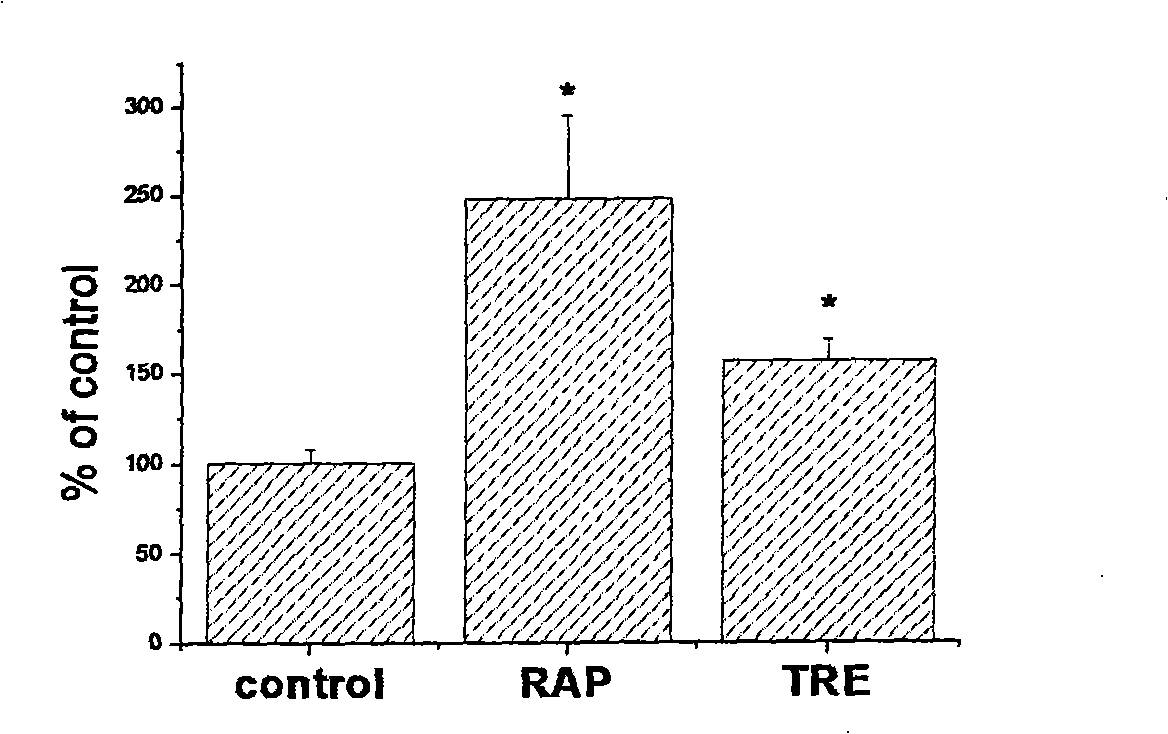
[0027] Example 1: Applying a compound that promotes the level of autophagy at the same time as non-viral vector-mediated gene transport [use cationic liposome as gene transport carrier, Luciferase as reporter gene, rapamycin (rapamycin) and Trehalose is a compound that increases the level of autophagy in cells].
[0028] Cells were seeded in 24-well cell culture plates at a density of approximately 3-5×10 4 / well, cultivated overnight, and set aside.
[0029] Prepare the transfection complex, the method is as follows: 1.5 μl of Lipofectamine2000 per well, dissolved in 100 μl DMEM (with serum and no antibiotics) medium, mixed well and incubated at room temperature for 5 min, DNA was 2 μg per well, dissolved in 100 μl DMEM (with serum and no antibiotics) Antibiotics) medium, mix well and incubate at room temperature for 5 minutes, then the two dissolve and mix evenly, and incubate at room temperature for 20-25 minutes.
[0030] The transfection process method is as follows: ov...
Embodiment 2
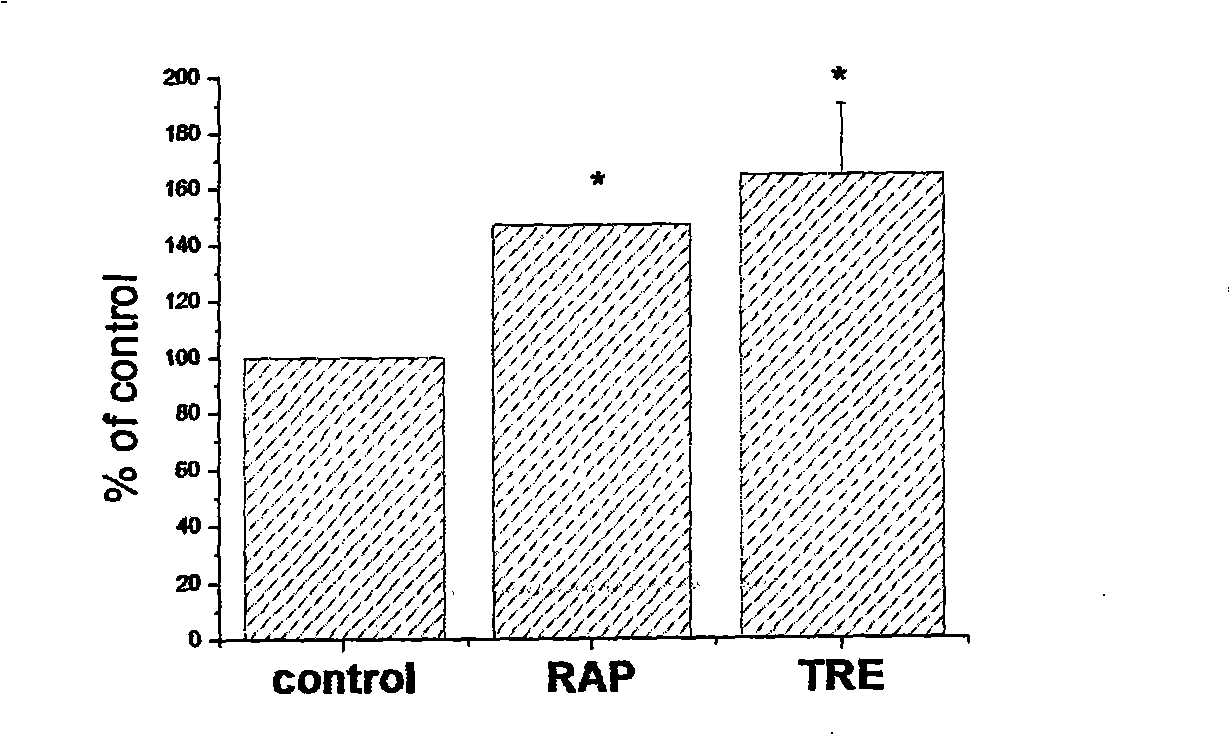
[0036]Example 2: Applying a compound that promotes the level of autophagy at the same time as the non-viral carrier-mediated gene transport [Using polyamine transfection reagents as the gene transport carrier, GFP as the reporter gene, rapamycin (rapamycin ) and trehalose (trehalose) as compounds that increase the level of autophagy].
[0037] The cells were seeded in a 24-well cell culture plate at a density of about 3-5×10 4 / well, cultured overnight, and ready for use (same as Example 1).
[0038] The preparation of the transfection complex and the transfection process were the same as in Example 1.
[0039] The detection method is as follows:
[0040] (1) The cells were collected after 24 hours of culture, and the cells were lysed with a cell lysate (Beiyuntian Company), boiled in water for 10 minutes, and then transferred to a nylon membrane (Amersham Company) by wet transfer after 12% SDS-PAGE electrophoresis. Western blot was performed using anti-GFP (Santa Cruz Compa...
Embodiment 3
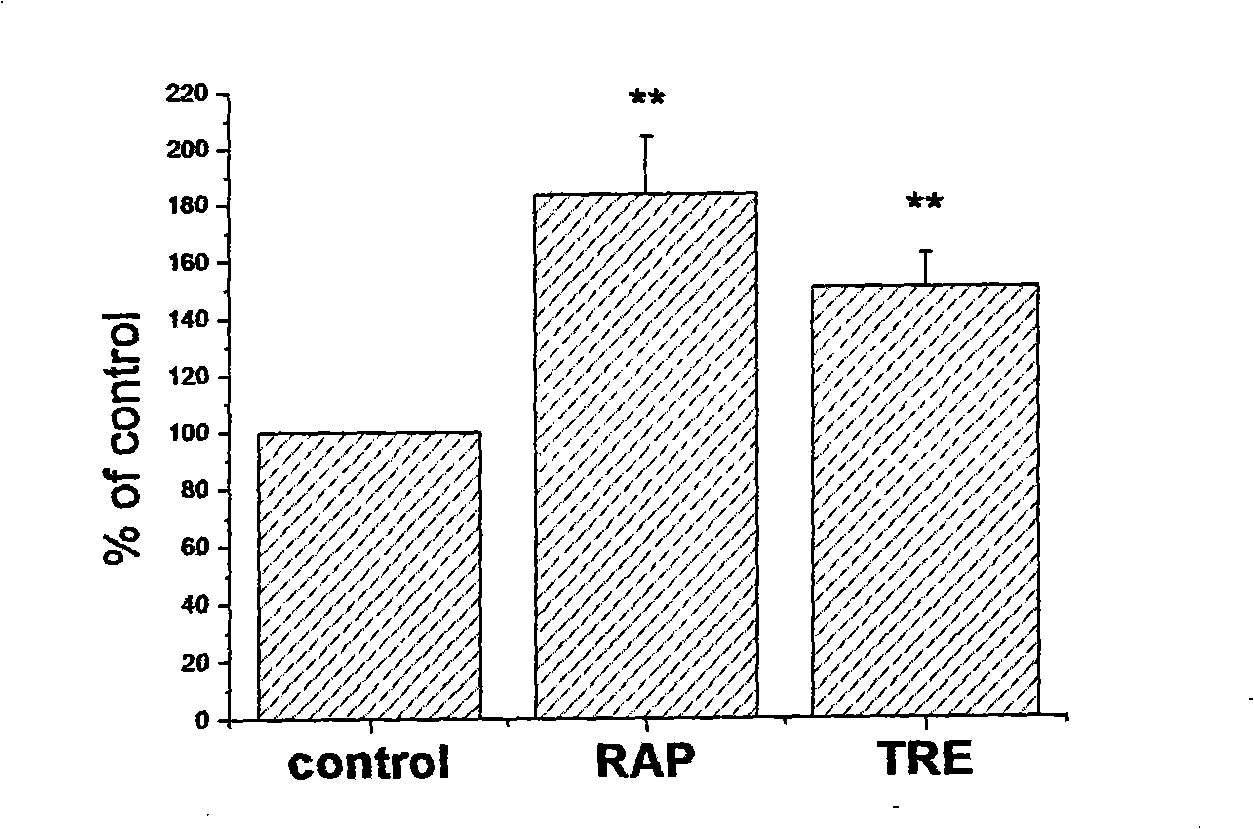
[0043] Example 3: Introducing a gene that increases the level of autophagy at the same time as the gene transport mediated by a non-viral carrier (liposome is used as a gene delivery carrier, Luciferase is used as a reporter gene, and p53 is used as a gene that improves the level of autophagy) .
[0044] Cells were seeded in 24-well cell culture plates at a density of approximately 3-5×10 4 / well, cultured overnight, ready for use (same as Example 1).
[0045] The preparation of the transfection complex was as follows: 1.5 μl of Lipofectamine2000 per well was dissolved in 100 μl of DMEM (containing serum and no antibiotics) medium, mixed well and incubated at room temperature for 5 min, the reporter gene DNA was 2 μg per well, and the p53 expression plasmid was 2 μg per well. Dissolve 1 μg of wells (the control group is p53 blank vector plasmid) in 100 μl DMEM (containing serum and no antibiotics) medium, mix well, incubate at room temperature for 5 minutes, and then the two ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com