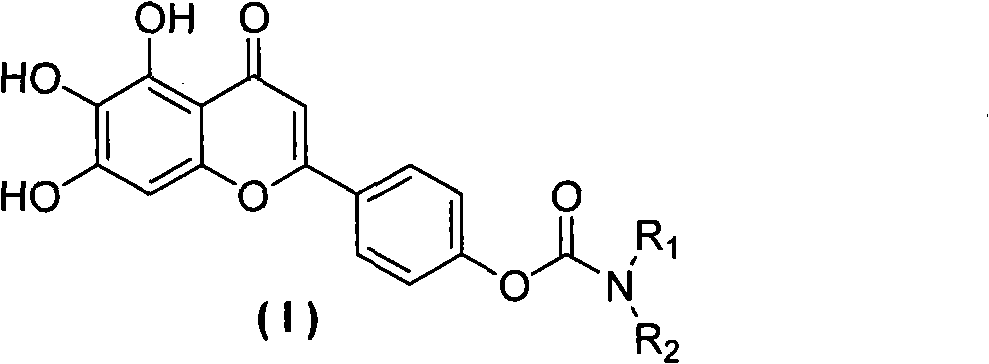
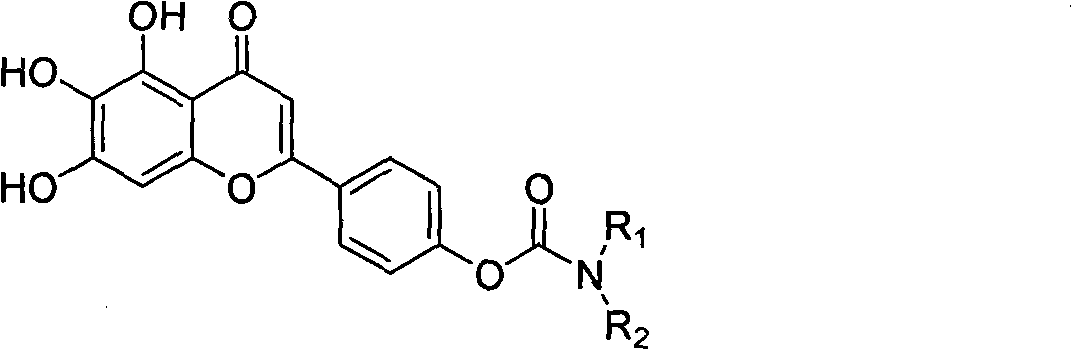
Scutellarein carbamate derivates, preparation method and application thereof
A technology of scutellarin aglycone and carbamate, applied in the field of scutellarin aglycone 4'-position carbamate derivatives and scutellarin aglycone derivatives, can solve the problem of poor solubility of scutellarin, Short half-life in vivo, serious side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
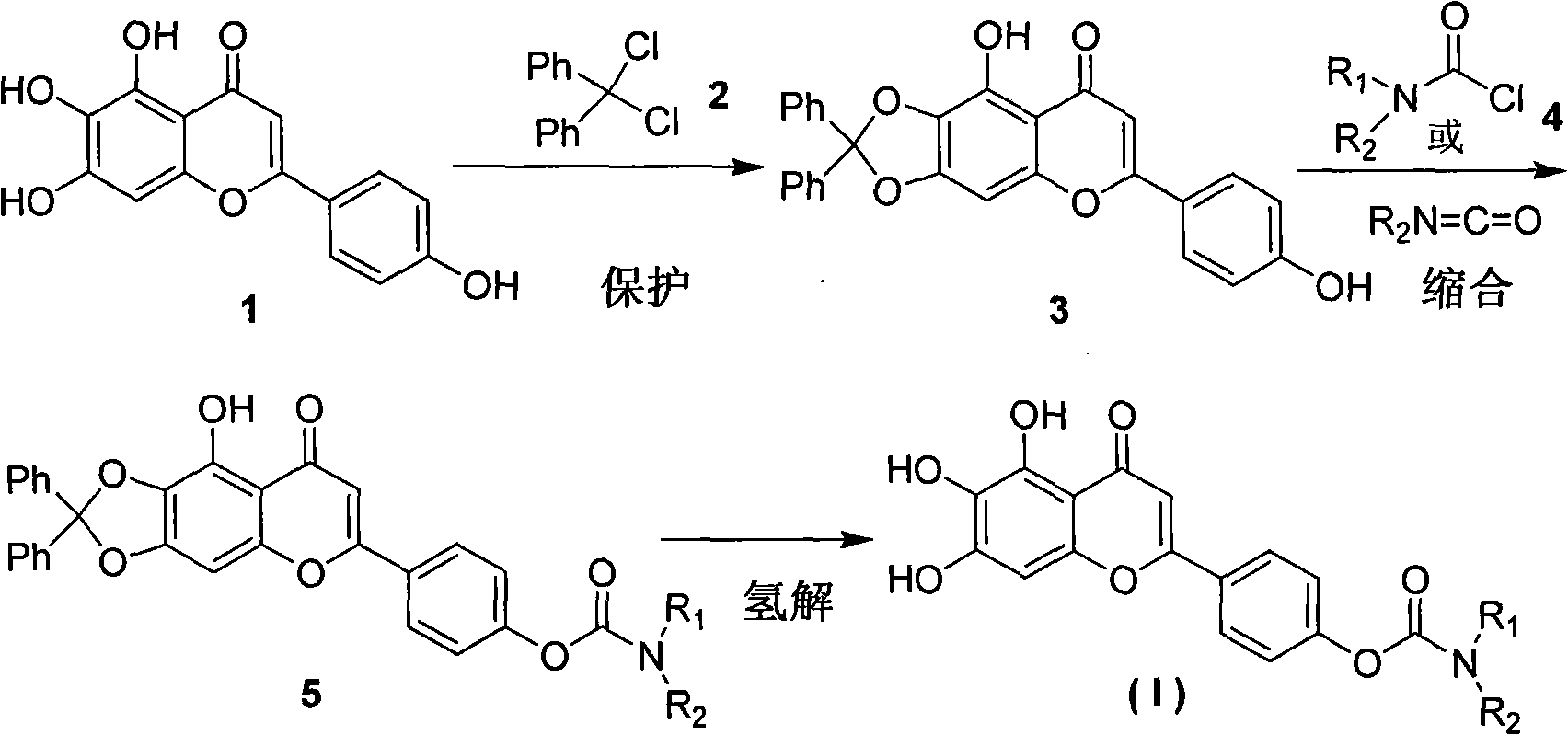
Embodiment 1
[0030] Preparation of 6,7-(diphenylmethylenedioxy)-5,4'-dihydroxyflavone (3)
[0031] Add 13.3 g (0.047 mol) of scutellarin aglycon and 16.7 g (0.070 mol) of diphenylmethylene chloride into the reaction flask in sequence, mix well, raise the temperature to 170°C for 1 hour under the protection of inert gas, and cool to room temperature Afterwards, add an appropriate amount of chloroform, suction filter while hot, evaporate the filtrate to remove the solvent under reduced pressure, and recrystallize the residue with chloroform to obtain 13.83 g of light brown needle crystals, mp: 250-252°C, yield 65.9%. 1 H NMR (DMSO-d 6 , 400MHz) δ: 13.18 (brs, 1H, 5-OH), 10.41 (brs, 1H, 4'-OH), 7.95 (d, J=8.8Hz, 2H, Ar'-H 3,5 ), 7.61(s, 1H, Ar-H 8 ), 7.59~7.56(m, 4H, Ph-H), 7.51~7.47(m, 6H, Ph-H), 6.94(d, J=8.8Hz, 2H, Ar’-H 2,6 ), 6.88(s, 1H, Ar-H 3 ); HR-TOFMS m / z: 449.1036 ([C 28 h 18 o 6 -H] + Calculated value: 449.1025).
Embodiment 2
[0033] Preparation of 6,7-(diphenylmethylenedioxy)-5-hydroxy-4'-(N-tetrahydropyrrolecarboxy)-flavone (5a)
[0034] Add 1.0g (2.21mmol) of intermediate 3, 0.35mmol of tetrahydropyrrolecarbonyl chloride, 30ml of N,N-dimethylformamide and 5ml of anhydrous pyridine into the reaction flask, stir and react at room temperature for 15 hours (the reaction process is followed by TLC ); after the reaction, add an appropriate amount of ice water, filter with suction, and wash the filter cake with a small amount of ice water, and the resulting crude product is purified by column chromatography (eluent: chloroform-ethyl acetate) to obtain 0.593 g of a light yellow powder solid, mp: 226~228°C, yield 49.0%. 1 H NMR (CDCl 3 , 400MHz) δ: 7.86 (d, J=8.8Hz, 2H, Ar'-H 3,5 ), 7.64~7.61(m, 4H, Ph-H), 7.41~7.37(m, 6H, Ph-H), 7.31(d, J=8.8Hz, 2H, Ar’-H 2,6 ), 6.65(s, 1H, Ar-H 8 ), 6.62(s, 1H, Ar-H 3 ), 3.56(t, J=6.8Hz, 2H, NCH 2 -H α ), 3.53(t, J=6.8Hz, 2H, NCH 2 -H β ), 1.92(t, J=6.8Hz, 2H, ...
Embodiment 3
[0036] Preparation of 6,7-(diphenylmethylenedioxy)-5-hydroxy-4'-(N,N-dimethylcarbamoyloxy)-flavone (5b)
[0037] The operation process is the same as in Example 2, except that tetrahydropyrrolecarbonyl chloride is replaced by N, N-dimethylcarbamoyl chloride to obtain 6,7-(diphenylmethylenedioxy)-5-hydroxyl-4'- (N,N-Dimethylcarbamoyloxy)-flavone is light yellow powder solid, mp: 230-232°C, yield 71.2%. 1 H NMR (CDCl 3 , 400MHz) δ: 7.86 (d, J=8.8Hz, 2H, Ar'-H 3,5 ), 7.64~7.61(m, 4H, Ph-H), 7.41~7.37(m, 6H, Ph-H), 7.28(d, J=8.8Hz, 2H, Ar’-H 2,6 ), 6.65(s, 1H, Ar-H 8 ), 6.62(s, 1H, Ar-H 3 ), 3.13 (s, 3H, CH 3 ), 3.04 (s, 3H, CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com