Compstatin and analogs thereof for eye disorders
A technology for eye diseases and analogs, which can be used in medical preparations containing active ingredients, metabolic diseases, drug combinations, etc., and can solve problems such as limited treatment options for ARMD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0089] overview
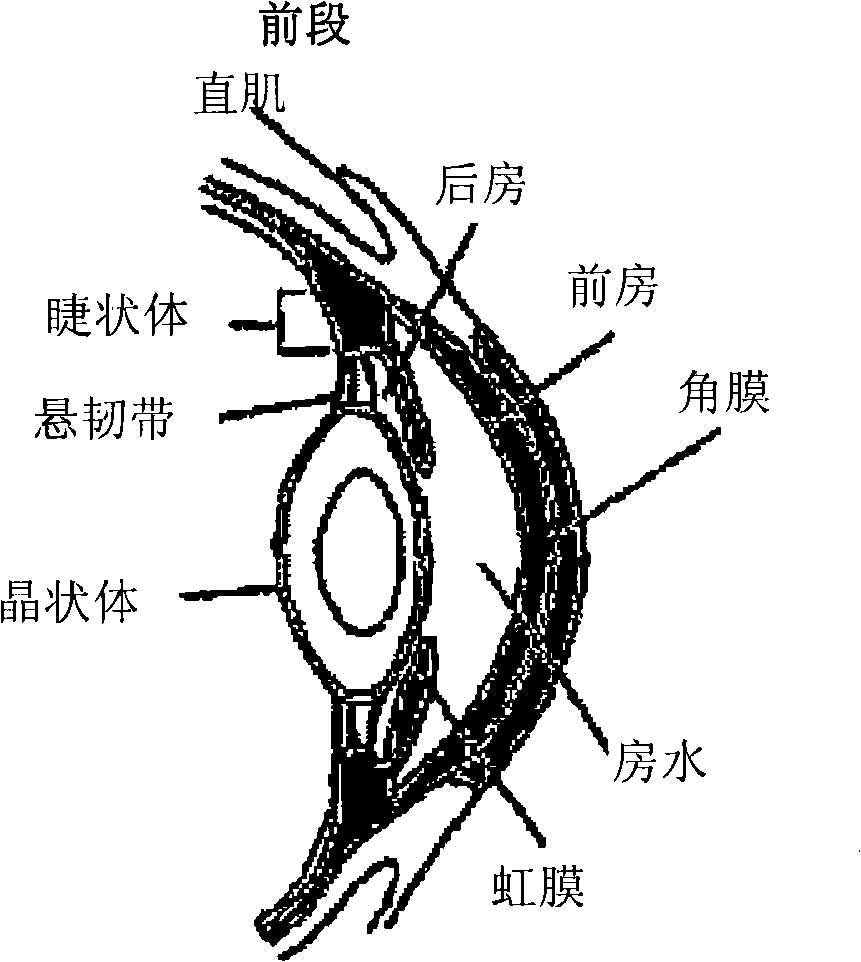
[0090] The present invention provides compositions and methods for treating ocular disorders characterized by macular degeneration, choroidal neovascularization, retinal neovascularization, ocular inflammation, or any combination of the foregoing. The phrase "characterized by" is intended to indicate that macular degeneration, CNV, RNV and / or ocular inflammation are characteristic (ie typical) features of the disorder. Macular degeneration, CNV, RNV and / or ocular inflammation can be defining and / or diagnostic properties of the condition. Exemplary conditions that are characterized by one or more of these properties and that can be treated with the compositions and methods of the invention include, but are not limited to, conditions associated with macular degeneration, diabetic retinopathy, retinopathy of prematurity, proliferative vitreoretinopathy, ocular Uveitis, keratitis and scleritis. As mentioned above, macular degeneration refers to various degenera...
example 1
[0309] Example 1: Prevention of Choroidal Neovascularization in a Mouse Model by Administration of Compstatin Analogs
[0310] Materials and methods
[0311] complement inhibitor
[0312] Recombinant VCP was produced in and purified from the Pichia pastoris expression system as described (Sahu, A et al., J. Immunol. 160, 5596-5604, 1998). VCP was dissolved in physiological saline at various concentrations.
[0313] figure 2 Compstatin analogs shown in were chemically synthesized and dissolved in saline with positions 4 and 9 altered relative to the compstatin peptide.
[0314] CNV induction in mice
[0315] C57BL / 6 mice (Jackson Laboratory) were anesthetized with a ketamine / xylazine (8:1) mixture and the pupils were dilated with a single drop of 1% tropicamide. Krypton red laser coagulation (50-μm spot size, 0.05 s duration, 250 mW) can be used to generate a laser spot around the optic nerve by using a hand-held coverslip as a contact lens. Bubble formation at the...
example 2
[0331] Example 2: Prevention of Choroidal Neovascularization in a Mouse Model by Administration of Compstatin Analogs
[0332] Example 1 was repeated using different compstatin analogs. Doses of 0.1-50 μg / eye were tested.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com