Non-serum medium suitable for microencapsulation CHO cell and uses thereof
A serum-free medium and microencapsulation technology, applied in tissue culture, animal cells, vertebrate cells, etc., can solve problems such as adverse effects, design of serum-free medium, and complex serum content, reducing the difficulty of separation and purification. cost, improved transplant biosafety, improved biosafety efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] ① The culture medium is prepared according to the following formula
[0030] The culture medium among the present invention is a mixture, is made up of commercialized DMEM / F12 standard culture medium (purchased from Sigma Company, specific composition sees Table 1) and following supplement:
[0031] Insulin 15mg / L
[0032] Soy Lecithin 40mg / L
[0033] Tween 80 20mg / L
[0034] Tropolone 1μM
[0035] Ferric ammonia (FAC) 0.1mg / L
[0036] Sodium selenite 2.5mg / L
[0037] β-Mercaptoethanol 20μM
[0038] L-Aspartic Acid 1mg / L
[0039] L-Glycine 8mg / L
[0040] L-Arginine 5mg / L
[0041] L-proline 1mg / L
[0042] L-histidine 4mg / L
[0043] L-Lysine 3mg / L
[0044] L-cysteine 10mg / L
[0045] L-Glutamine 100mg / L
[0046] All of the above substances are analytically pure chemical reagents.
[0047] The culture medium of the present invention is prepared by a conventional mixing method.
[0048] The culture medium described in the present invention can be used accord...
Embodiment 2
[0056] ① The culture medium is prepared according to the following formula
[0057] Add the following supplements to 1L DMEM / F12 medium, and then add this medium to the culture flask, the cell density of CHO cells after microencapsulation is 2×10 6 cells / ml, cultivated for 7 days.
[0058] Insulin 5mg / L
[0059] Soy Lecithin 10mg / L
[0060] Tween 80 20mg / L
[0061] Tropolone 3μM
[0062] Ferric ammonia (FAC) 1mg / L
[0063] Sodium Selenite 10mg / L
[0064] β-Mercaptoethanol 50μM
[0065] L-Aspartic Acid 5mg / L
[0066] L-Glycine 20mg / L
[0067] L-Arginine 10mg / L
[0068] L-Proline 5mg / L
[0069] L-histidine 10mg / L
[0070] L-Lysine 8mg / L
[0071] L-cysteine 20mg / L
[0072] L-Glutamine 400mg / L
[0073] 2. Preparation of microencapsulated CHO cells (same as Example 1)
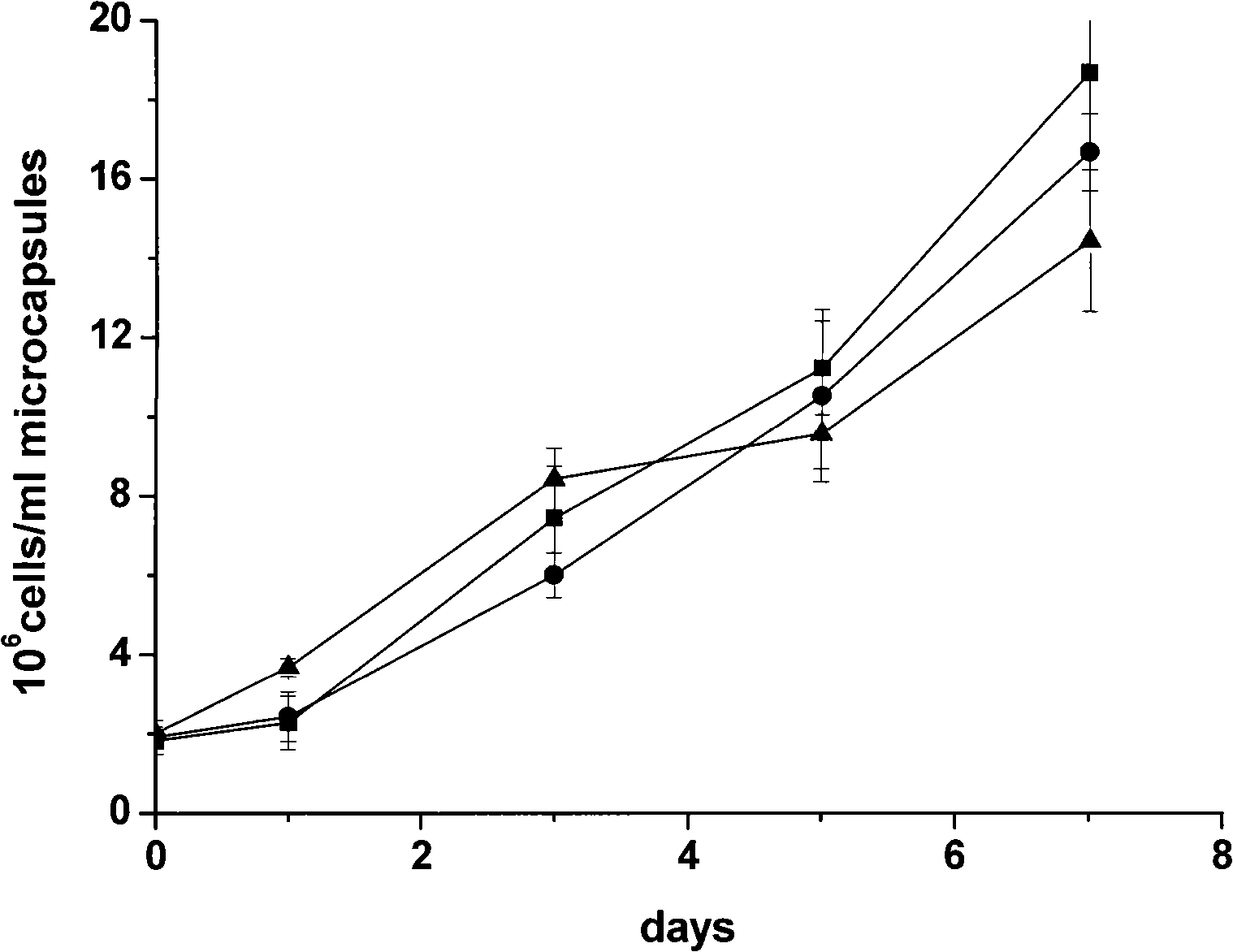
[0074] ③ microencapsulated cells at 37°C, 5% CO 2 The cell density after 7 days in the incubator was 16×10 6 cells / ml microcapsules.
Embodiment 3
[0076] ① The culture medium is prepared according to the following formula
[0077] Add the following supplements to 1L DMEM / F12 medium, and then add this medium to the culture flask, the cell density of CHO cells after microencapsulation is 2×10 6 cells / ml, cultivated for 7 days.
[0078] Insulin 10mg / L
[0079] Soy Lecithin 30mg / L
[0080] Tween 80 40mg / L
[0081] Tropolone 5μM
[0082] Ferric Ammonia (FAC) 0.5mg / L
[0083] Sodium selenite 8mg / L
[0084] β-Mercaptoethanol 30μM
[0085] L-Aspartic Acid 3mg / L
[0086] L-Glycine 3mg / L
[0087] L-Arginine 1mg / L
[0088] L-proline 10mg / L
[0089] L-histidine 1mg / L
[0090] L-Lysine 10mg / L
[0091] L-cysteine 3mg / L
[0092] L-Glutamine 200mg / L
[0093] 2. Preparation of microencapsulated CHO cells (same as Example 1)
[0094] ③ microencapsulated cells at 37°C, 5% CO 2 The cell density after 7 days in the incubator was 15×10 6 cells / ml microcapsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com