Multi-type HCV-E1 epitope complex immunogen, encoding gene and application thereof
A compound antigen and epitope technology, applied in the field of compound immunogens, to overcome the effect of variation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Screening of HCV E1 epitope representative sequences
[0061] 1. Screening of HCV E1 neutralizing epitopes In the HCV Immunology Database, there is a summary of HCV E1 antibody epitopes, among which the only human antibodies are H111 and U1 / 30-33 series antibodies. U1 / 30-33 series antibodies are early years (Siemoneit K, Cardoso Mda S, et al. Human monoclonal antibodies for the immunological characterization of a highly conserved protein domain of the hepatitis Cvirus glycoprotein E1. Clin Exp Immunol. 1995; 101(2): 278-83) The human monoclonal antibody studied was the result of screening with synthetic peptides (314-330), but the binding effect of this antibody on recombinant E1 antigen was not clear, and whether it had neutralizing activity was not studied. Most other antibodies are obtained in animal experiments. Therefore, the present invention selects the action epitope of the neutralizing antibody H111. In addition, there are A4(m) and 159 antibody epi...
Embodiment 2
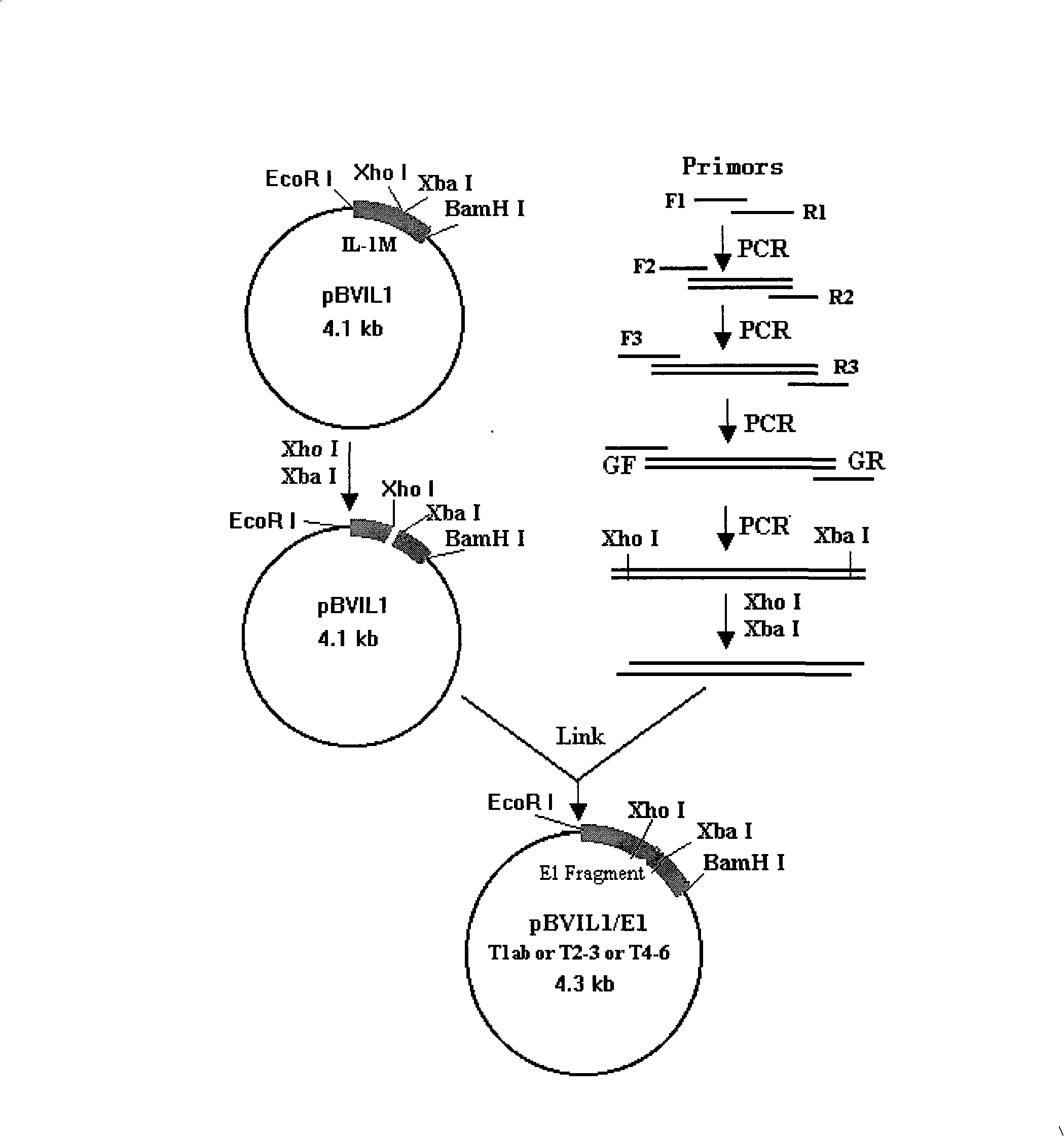
[0065] The construction of embodiment 2 recombinant polytype HCV E1 expression plasmid
[0066] The representative sequence of HCV E1 has been selected above, and the universal TH epitope sequence PADRE has also been determined, but they are all amino acid sequences. If they are to be expressed in the form of recombinant proteins, they must first be converted into gene sequences and cloned into expression vectors. The following studies were carried out for this purpose:
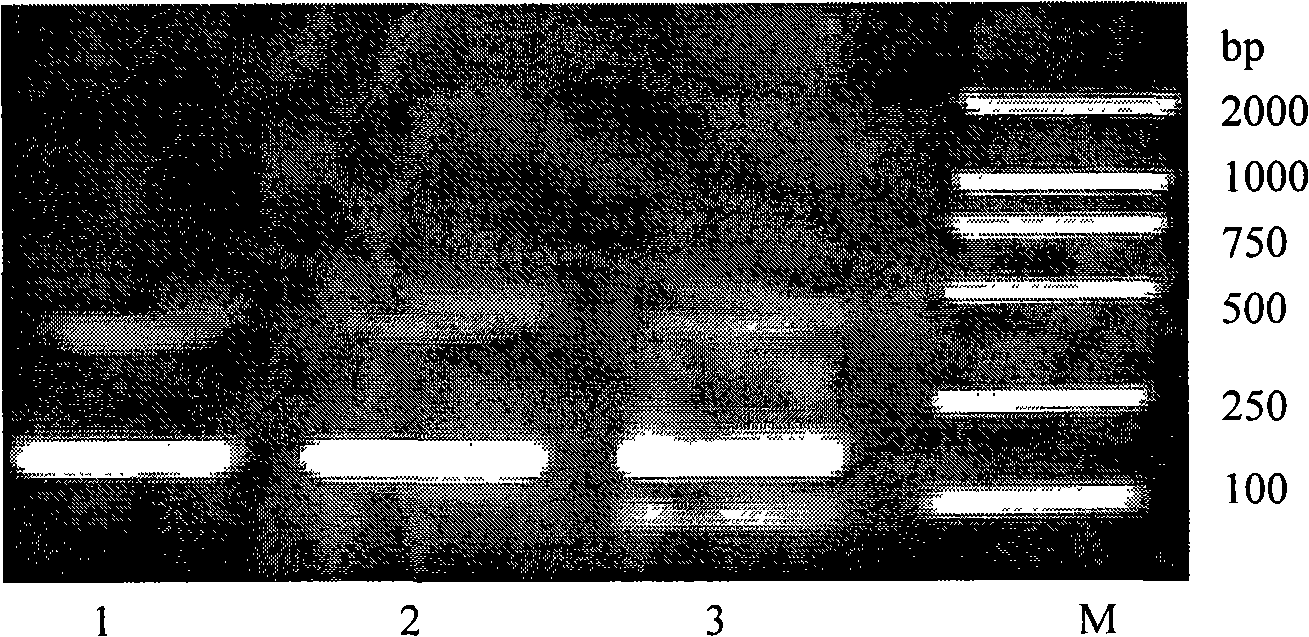
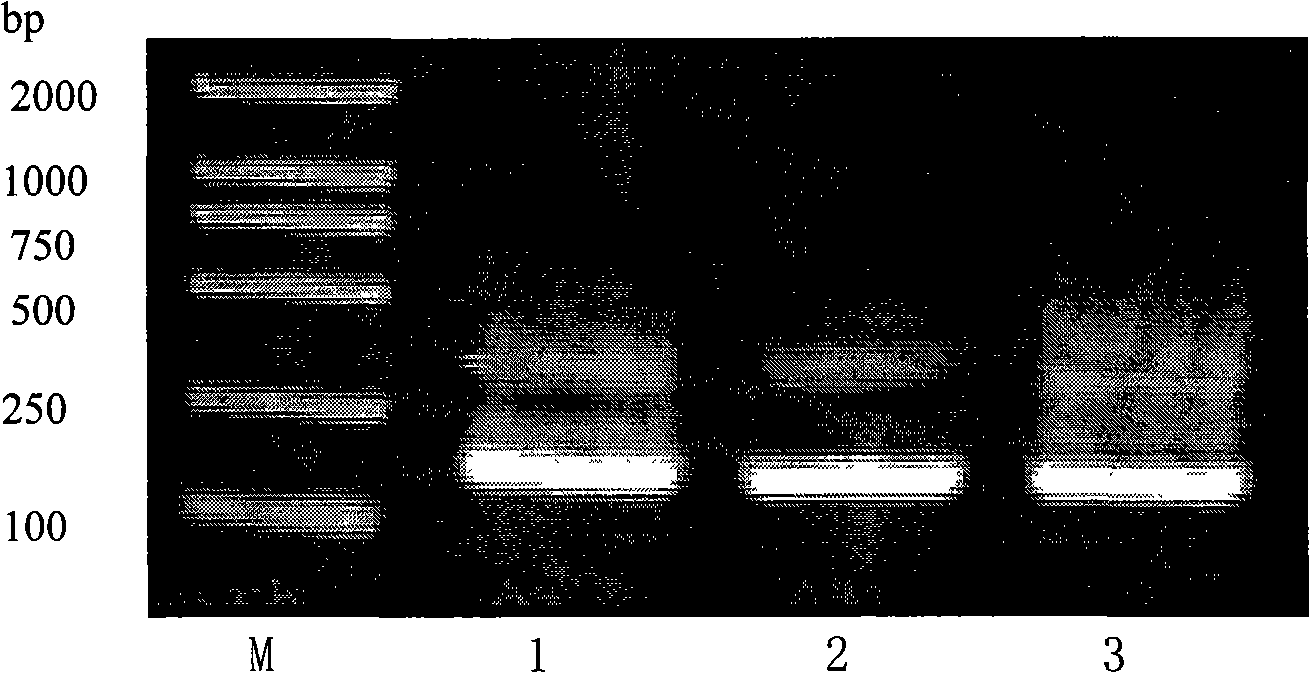
[0067] 2.1 The design of synthetic gene primers: According to the sequence 10, 11, 12, the gene sequence was deduced according to the biased codon of Escherichia coli, and three sets of primers for gene synthesis were respectively designed, and connecting arms were added to the 5' and 3' ends respectively, In order to add enzyme cutting sites after amplification with universal primers, it can be connected with the carrier. The specific sequences of the three sets of designed primers are shown in Sequences 13...
Embodiment 3
[0160] Example 3 Recombinant Multitype HCV / E1 Epitope Compound Antigen Immunogenicity Analysis
[0161] Through the above-mentioned Examples 1 and 2, the recombinant multi-type HCV E1 complex epitope antigen has been obtained, but whether the antigen is immunogenic, that is, whether it can effectively stimulate the body to produce antibodies, further research is needed. protocol, mice were immunized three times. Blood was collected half a month after each immunization to determine the antibody level of the immunized mice. In order to avoid the interference of part of the IL-1 molecule fused in the antigen when measuring the antibody titer, the pBV / E1s-PADRE expression plasmid was also constructed, which can express multitype HCV / E1 epitopes without IL-1 The composite antigen (refer to the above examples for the preparation steps, except that IL-1 was not introduced during the construction of the plasmid) was used as the antigen for antibody determination. The specific operat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com