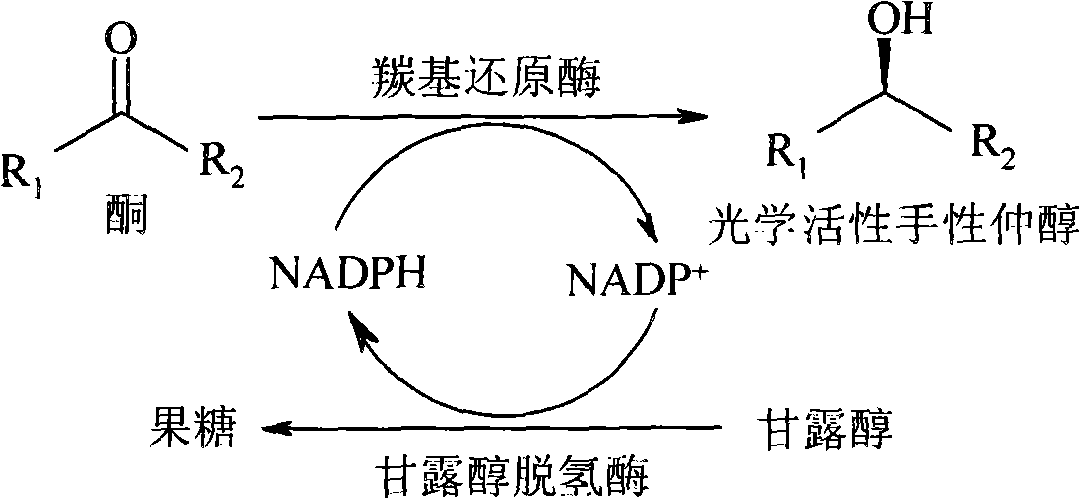
Method for preparing optical activity chirality secondary alcohol with rhodotorula reductase formulation
An optically active, reductase technology, applied in microorganism-based methods, biochemical equipment and methods, fermentation, etc., can solve the problems of limited large-scale industrial application, high price, etc., achieve good industrial application development prospects, easy to prepare, The effect of high substrate concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
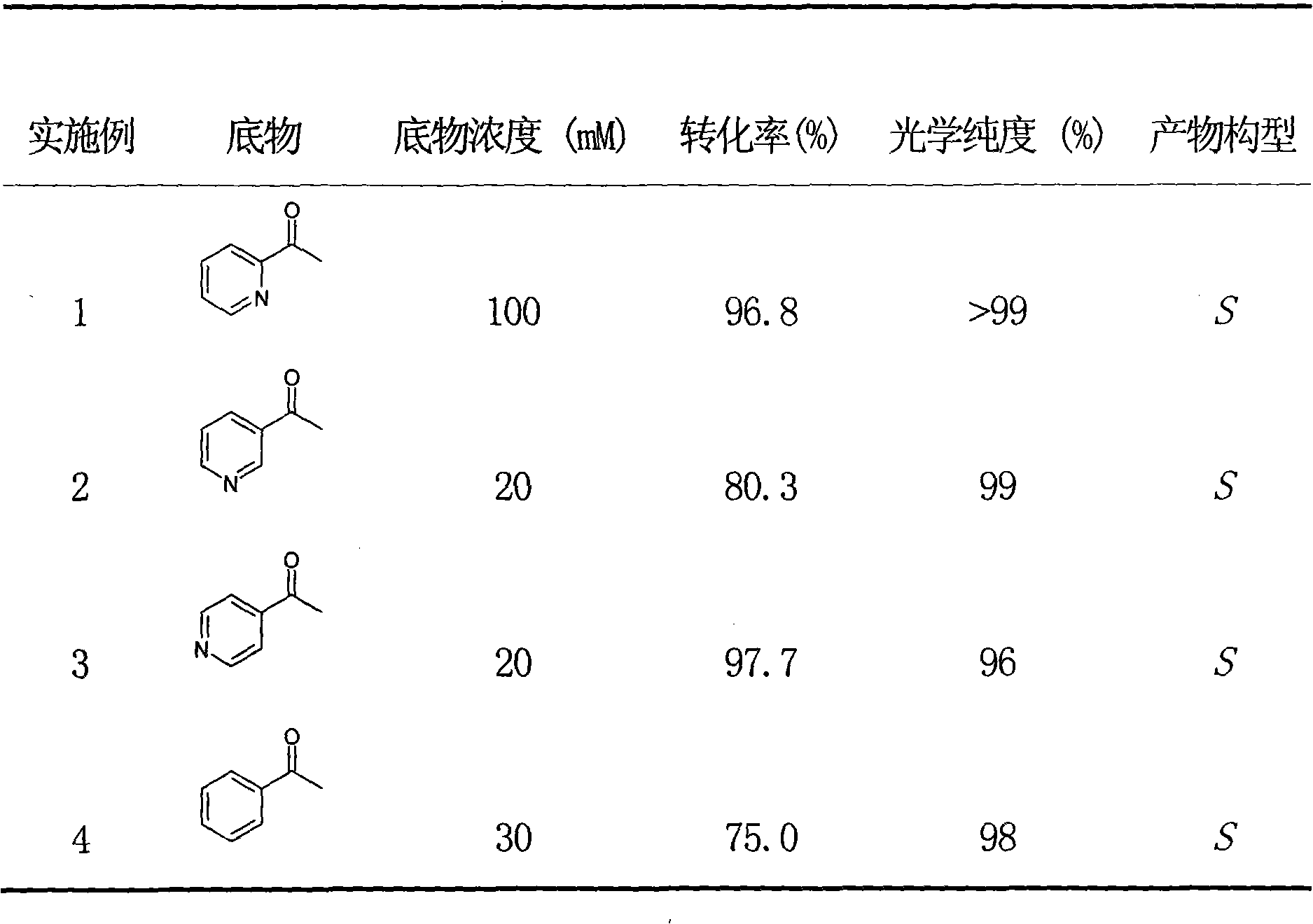
[0023] Take 5mg of Rhodotorula reductase crude enzyme preparation, add to 1ml NaHC 3 -Na 2 CO 3 In the buffer (100mM, pH 9.0), various substrates of different concentrations were added, such as acetylpyridine, benzene ring or methyl-substituted acetophenone derivatives, and 20 μl of NADPH solution with a concentration of 10 mM and 72 mg of mannitol were added, Shake the reaction on a 30 °C thermostatic shaker. After reacting for 24 hours, extract with an equal volume of ethyl acetate, add anhydrous sodium sulfate to the extract and dry it, and analyze the reaction conversion rate and the optical purity of the product by gas chromatography. The results are shown in Table 1.
[0024] Table 1 The crude enzyme preparation of Rhodotorula catalyzes the asymmetric reduction of acetylpyridine and aromatic ketones
[0025]
[0026]
Embodiment 9
[0028] Take 50mg of Rhodotorula reductase crude enzyme preparation, add to 10ml NaHCO 3 -Na2 CO 3 Buffer (100mM, pH 9.0), add 0.12g 2-acetylpyridine (final concentration is 100mM), add 0.1ml concentration of 10mM NADP + 0.9 g of mannitol was added to the solution, and the reaction was shaken on a constant temperature shaker at 30° C., and samples were taken intermittently to measure the conversion rate of the reaction. After 1 hour of reaction, the conversion rate was 17.1%. After 24 hours, the substrate was completely converted, and 0.098 g of the product (S)-2-pyridine-1-ethanol was isolated with an optical purity of >99%.
Embodiment 10
[0030] Take 10mg of partially purified Rhodotorula reductase preparation, add to 50ml NaHCO 3 -Na 2 CO 3 Buffer (100mM, pH9.0), add 0.605g 2-acetylpyridine (final concentration is 100mM), add 1ml concentration of 10mM NADP + 3.6 g of mannitol was added to the solution, and the reaction was shaken on a constant temperature shaker at 30° C., and samples were taken intermittently to measure the conversion rate of the reaction. After 1 hour of reaction, the conversion rate was 41.5%. By 5 hours, the substrate had been completely reduced and transformed. Add 50ml of ethyl acetate to extract the product (S)-2-pyridine-1-ethanol in the reaction solution, let stand to separate layers, take out the solid organic phase of ethyl acetate, and add 50ml of ethyl acetate to the water phase to extract again, so Repeat 4 times. Combine the ethyl acetate organic phases, add an appropriate amount of anhydrous sodium sulfate to dry overnight, filter, and remove the ethyl acetate in the filtra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com