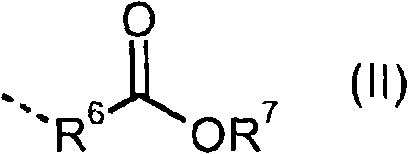
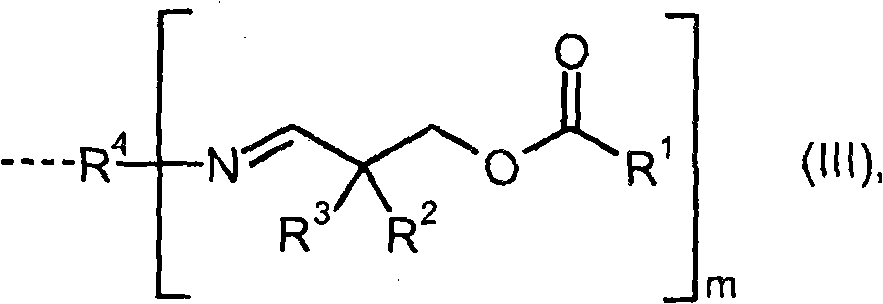
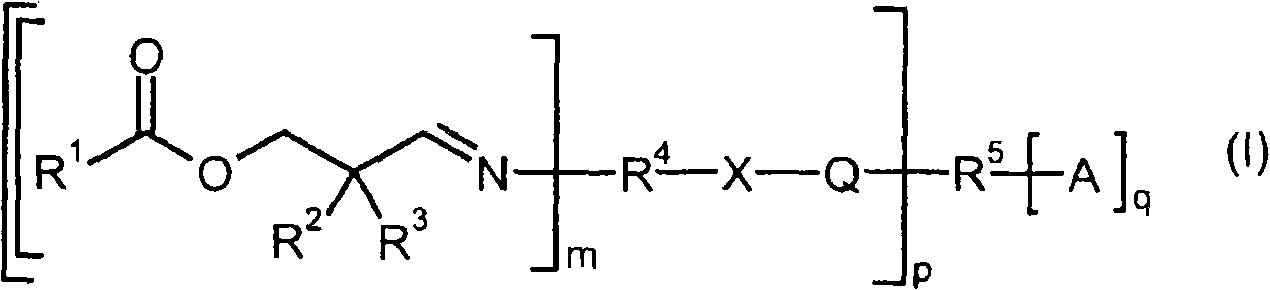Compounds containing aldimine
A technology containing aldimines and compounds, which is applied in the field of aldimines and aldimine-containing compounds, and can solve problems such as limited use, unusable compositions, limited storage stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0197] Example 1 (aldimine-containing compound AV1)
[0198] In a round bottom flask, add 1.74 g (13.9 mmol NCO) 4,4'-diphenylmethane-diisocyanate (MDI; 44MC L, Bayer) and heated to 50°C. From the dropping funnel, 10.00 g (13.9 mmol) of aldimine AL3 are added within 5 minutes with good stirring, and the mixture is stirred at 50° C. for one hour. A colorless, viscous liquid at room temperature, a clear and odorless liquid with an amine content of 2.37 mmol NH 2 / g, which reacts neutrally on wetted pH test paper.
[0199] IR: 3300(N-H), 2952sh, 2922, 2851, 1735(C=O), 1664(C=N), 1647sh, 1595, 1527sh, 1513, 1466, 1416, 1395, 1375, 1305, 1244, 1215, 1196 , 1162, 1112, 1056, 1018, 1000, 939, 918sh, 851, 813, 777, 751, 721.
Embodiment 2
[0200] Embodiment 2 (compound AV2 containing aldimine)
[0201] In a round bottom flask, add 3.47g (27.7mmol NCO) 4,4'-diphenylmethane-diisocyanate (MDI; 44MC L, Bayer) and heated to 50°C. From the dropping funnel, 10.00 g (13.9 mmol) of aldimine AL3 are added within 5 minutes with good stirring, and the mixture is stirred at 50° C. for one hour. This gave a pale yellow, viscous liquid at room temperature, a clear and odorless liquid which reacted neutrally on moistened pH paper.
[0202] IR: 3308(N-H), 2954sh, 2922, 2852, 2266(N=C=O), 1735(C=O), 1665(C=N), 1596, 1526sh, 1514, 1467, 1415, 1395, 1374, 1306, 1244, 1216, 1197, 1162, 1110, 1059, 1018, 1000, 940, 918sh, 854, 813, 781, 751, 721.
Embodiment 3
[0203] Embodiment 3 (compound AV3 containing aldimine)
[0204] In a round bottom flask, charge 12.94 g (103.4 mmol NCO) 4,4'-diphenylmethane-diisocyanate (MDI; 44MC L, Bayer) and heated to 50°C. From the dropping funnel, 42.16 g (51.7 mmol) of aldimine AL4 are added within 10 minutes with good stirring, and the mixture is stirred at 50° C. for one hour. This gave a yellowish, viscous liquid at room temperature, a clear and odorless liquid which reacted neutrally on moistened pH paper.
[0205]IR: 3336(N-H), 2922, 2852, 2265(N=C=O), 1736(C=O), 1666(C=N), 1640, 1594, 1513, 1488, 1465, 1416, 1394, 1373, 1307, 1237, 1169, 1110, 1065, 1018, 1000sh, 932, 918sh, 848, 812, 776, 754, 723.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com