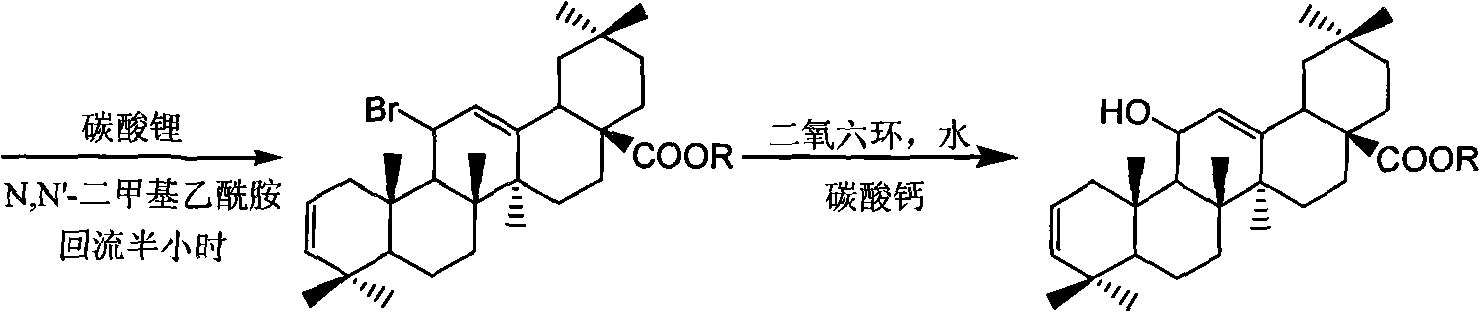
11-brominated-12-oleanane type pentacyclic triterpenoid and application thereof
A technology of pentacyclic triterpenoids and ene oleananes, which is applied in the field of polyoxidatively substituted pentacyclic triterpene derivatives and their preparation, can solve the problem of high incidence of adverse reactions of non-steroidal anti-inflammatory drugs, liver function Failure, aggravation of peptic ulcer and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0049] Preparation Example 1: Preparation of Compound Ii
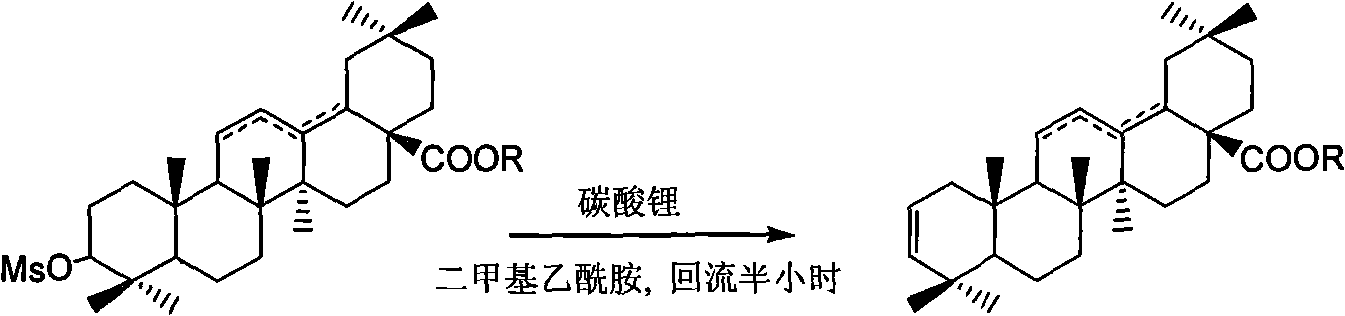
[0050] The compound 3-methylsulfonyloxy-oleanane-28-acid-12-ene (1.0mmol) was dissolved in 5 ml of DMAC (N,N'-dimethylacetamide), and lithium carbonate ( 2.0 mmol). The reaction mixture was heated to reflux for half an hour. After cooling, excess lithium carbonate was filtered off, the filtrate was added to 10 ml of water, extracted with ether, and the organic phase was washed with water until neutral. Dry over anhydrous sodium sulfate. Silica gel column chromatography (eluent: petroleum ether / ethyl acetate: 8 / 1) yielded oleanane-28-acid-2,12-diene as a white solid with a yield of 82%.
[0051] In 20 ml of acetic acid (containing 5% acetic anhydride), the compound oleanane-28-acid-2,12-diene (0.82 mmol) and chromium trioxide (4.92 mmol) were added, and stirred overnight at room temperature. The reaction compound was poured into 20 ml of water, and extracted with chloroform. The organic phase was washed with satura...
preparation example 2~ preparation example 22
[0053] According to the method of Preparation Example 1, the compounds of Preparation Example 2 to Preparation Example 22 shown below were prepared:
preparation example 2
[0054] Preparation Example 2: Preparation of oleanane-28-acid-2,11,13(18)-triene (Ia), C 30 h 44 o 2 , MS: ESI m / e436 (M + ); Rf (petroleum ether / chloroform / methanol: 4 / 4 / 0.6): 0.63, 1 H NMR (400MHz, CDCl 3 )δ: 2.51(brd, 1H, J=14.2Hz, H-19), 5.31-5.43(m, 3H, H-2, H-3, H-12), 6.35(d, 1H, J=10.4Hz , H-11).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com