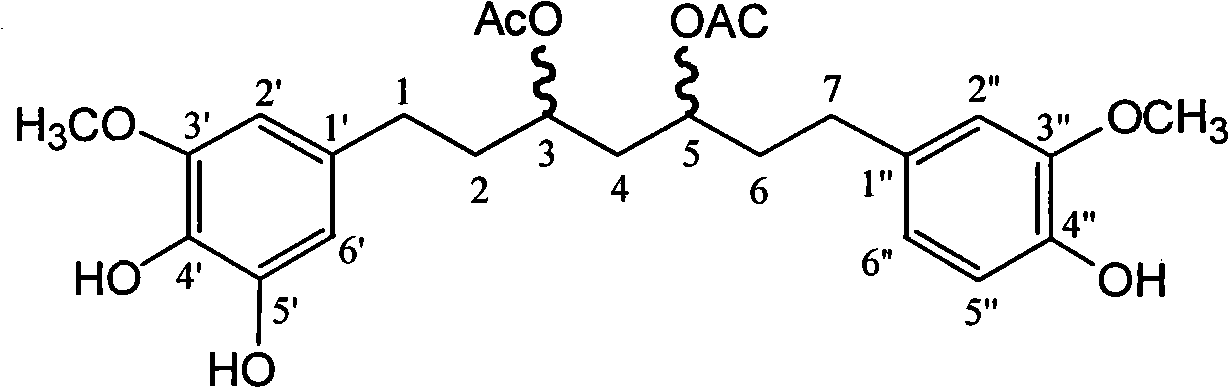
Pharmaceutical use of diacetyl diphenyl sulphone heptane in preventing and controlling chronic marrow-derived leukocythemia
A technology of diacetyl and leukemia, which is applied in the field of natural medicinal chemistry and pharmacology, and can solve the problems of single target, poor adverse reaction reversal effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The phenolic diphenylheptane compound 3,5-diacetyl-1-(3-methoxyl-4,5-dihydroxyphenyl)-7-(4-hydroxyl-3-methyl) involved in the present invention The extraction and preparation methods of oxyphenyl)heptane include: ultrasonic extraction of the rhizomes, tubers, leaves, and flowers of Zingiberaceae plants through alcohol water or ketone water, macroporous resin enrichment, normal phase and reverse phase silica gel column chromatography purification made. Extraction method of the present invention comprises the following steps:
[0017] (a) The rhizomes, tubers, leaves, and flowers of Zingiberaceae plants that are dried and ground by cold immersion or ultrasonic extraction with alcohol water or ketone water, and evaporate the solvent under reduced pressure to obtain an extract;
[0018] (b) Dissolve with ethanol or methanol, then go through macroporous resin column chromatography, wash with water to remove impurities, and then use alcohol-water system gradient elution to o...
Embodiment 1
[0024] Example 1 : Separation and preparation of 3,5-diacetyl-1-(3-methoxy-4,5-dihydroxyphenyl)-7-(4-hydroxyl-3-methoxyphenyl)heptane in ginger and purification method
[0025] The crude drug of ginger was collected from Luoping County, Yunnan Province, and the crude drug specimen was identified by Professor Zhang Rongping of Kunming Medical College. Dried ginger rhizomes (5.5 kg) are ground and soaked with 10 times the amount of 95% ethanol, and extracted with ultrasound for 3 hours each time. After the solvent was evaporated under reduced pressure, the macroporous resin column was chromatographed, and ethanol-water (0:100-100:0) gradient elution was used. According to the detection results of thin-layer chromatography, the fractions of the same components were combined. The fractions eluting with ethanol-water (65:35) were collected, and the solvent was evaporated under reduced pressure to obtain 3.3 g of a brown solid. They were then subjected to column chromatography on...
Embodiment 2
[0027] Example 2 : Separation and preparation of 3,5-diacetyl-1-(3-methoxy-4,5-dihydroxyphenyl)-7-(4-hydroxyl-3-methoxyphenyl)heptane in Wenyujin and purification method
[0028] The rhizome of Wenyujin is collected from the south of Ruian, Zhejiang Province, and the crude drug is identified by Professor Huang Kexin of Wenzhou Medical College.
[0029] Dried warm turmeric rhizomes (6.0 kg) are ground and soaked with 10 times the amount of 95% ethanol, and extracted with ultrasound for 3 hours each time. After the solvent was evaporated under reduced pressure, the macroporous resin column was chromatographed, and ethanol-water (0:100-100:0) gradient elution was used. According to the detection results of thin-layer chromatography, the fractions of the same components were combined. The fractions eluting with ethanol-water (65:35) were collected, and the solvent was evaporated under reduced pressure to obtain 5.1 g of a brown solid. They were then subjected to column chromat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com