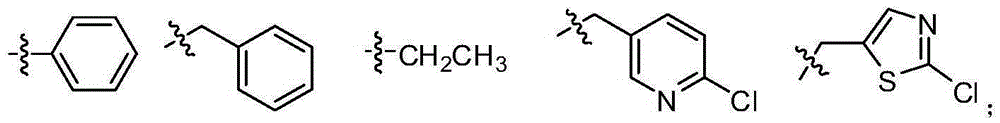Conjugated diene derivatives, preparation method thereof and use as anticancer drug
A technology of conjugated dienes and derivatives, which is applied in drug combinations, antineoplastic drugs, organic chemistry, etc., can solve the problems of prolonging the survival time of cancer patients and not being able to completely cure cancer, and achieve the ability to inhibit the growth of tumor cells. The effect of being simple and easy to implement and having broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
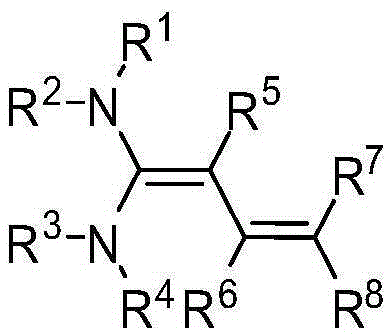
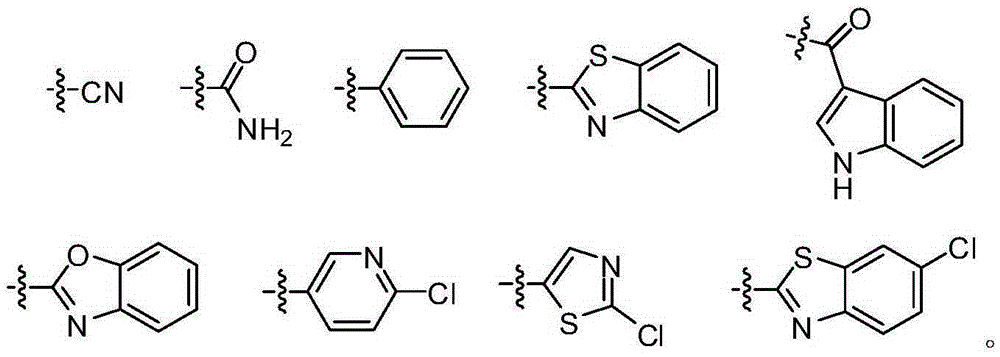
[0032] The structure of the partial conjugated diene derivative of the present invention is one of the specific compounds listed in Table 1:
[0033] Table 1: List of representative compound structures of general formula I
[0034]
[0035]
[0036]
[0037]
[0038]
Embodiment 2
[0040] Compound 2-(benzo[d]thiazol-2-yl)-4-(1-(phenyl)imidazoline-2-ylidene)-4-nitrobutyl- 2-alkene nitrile, its preparation method is:
[0041]
[0042] Using N-phenylethylenediamine as the starting material, referring to the method disclosed in the patent WO2004058714A1, successively undergoes a substitution reaction with 1,1-dithiomethyl-2-nitroethylene, and then reacts with N,N-dimethyl The intermediate 2-(1-(phenyl)imidazolin-2-ylidene)-2-nitroacetaldehyde (M-1) was prepared by condensation reaction of methyl formamide dimethyl acetal and alkaline hydrolysis reaction. Then weigh M-1, that is, 2-(1-(phenyl)imidazoline-2-ylidene)-2-nitroacetaldehyde 0.12g (0.5mmol) and dissolve it in 10mL absolute ethanol. Add 0.096g (0.55mmol) of 2-cyanomethylbenzothiazole (compound A) and a catalytic amount of potassium hydroxide, stir the reaction mixture at room temperature for 30 minutes, then slowly heat to 40-45°C, keep warm for reaction, and monitor by TLC until the reaction is...
Embodiment 3
[0044] Compound 2-(benzo[d]oxazol-2-yl)-4-(1-((6-chloropyridin-3-yl)methyl)imidazoline-2 numbered as I-37 in Example 1 -subunit)-4-nitrobut-2-enenitrile, its preparation method is:
[0045]
[0046] Using 2-chloro-5-chloromethylpyridine as the starting material, it undergoes the substitution reaction with ethylenediamine, the substitution reaction with 1,1-dithiomethyl-2-nitroethylene, and then with N,N -Dimethylformamide dimethyl acetal condensation reaction, alkaline hydrolysis reaction to prepare intermediate 2-(1-((6-chloropyridin-3-yl)methyl)imidazoline-2-ylidene)-2 - Nitroacetaldehyde (M-2). Then weigh M-2, 0.14g (0.5mmol) of 2-(1-((6-chloropyridin-3-yl)methyl)imidazolin-2-ylidene)-2-nitroacetaldehyde in 10mL In absolute ethanol, at room temperature, 0.087 g (0.55 mmol) of 2-cyanomethylbenzoxazole (compound B) and a catalytic amount of piperidine were added successively, and the reaction mixture was first stirred at room temperature for 30 minutes, and then slowly h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com