Felodipine controlled release formulation and preparation method thereof
A technology for controlled release preparations and preparations, which is applied to non-active ingredients medical preparations, pharmaceutical formulas, and medical preparations containing active ingredients, etc. Patient's inconvenience and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of felodipine controlled-release preparation
[0045] Prescription (per 1000 tablets):
[0046] Tablet core: Felodipine 5g, HPMC K100LV19.5g, HPMC K100M6.5g, sodium chloride 22.75g, lactose 9.75g, crospovidone 6.5g, silicon dioxide 0.7g, medicinal ethanol 38.9g;
[0047] Coating layer: HPMC K100LV 180g, lactose 270g, magnesium stearate 2.25g, silicon dioxide 4.5g, medicinal ethanol 305g.
[0048] Preparation:
[0049] (1) Tablet cores are granulated separately and pressed into tablets (φ5.5mm shallow concave punch);
[0050] (2) The coating layer is granulated separately;
[0051] (3) Press-coated chips (φ12mm shallow concave punch): first fill part of the coating layer particles in the die hole, then place the tablet core in the center of the die hole, add the remaining coating layer particles, and then press to form a chip-coated chip.
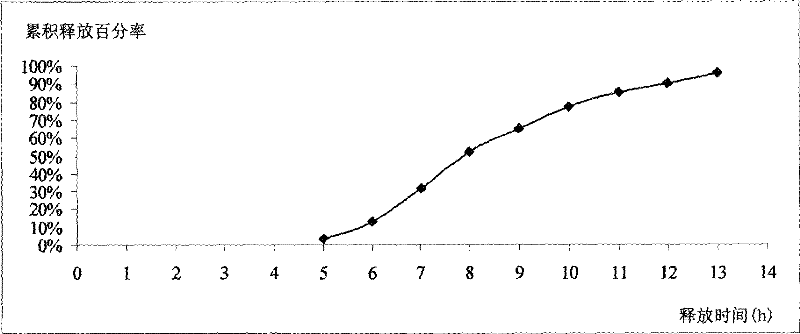
[0052] Determination of in vitro release: according to the national drug standard WS 1 -(X-152)-2005Z Felodipine Su...
Embodiment 2
[0055] Preparation of felodipine controlled-release preparation
[0056] Prescription (per 1000 tablets):
[0057] Tablet core: Felodipine 5g, HPMC K100LV 19.5g, lactose 32.5g, crospovidone 13g, silicon dioxide 0.7g, medicinal ethanol 37.1g;
[0058] Coating layer: HPMC K100LV 180g, lactose 270g, magnesium stearate 2.25g, silicon dioxide 4.5g, medicinal ethanol 305g.
[0059] Preparation method: as described under Example 1.
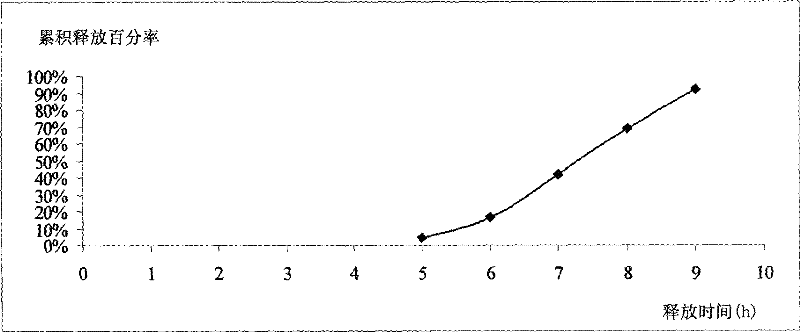
[0060] In vitro release assay: as described under Example 1. The experimental data are shown in Table 2 and attached figure 2 .
[0061] The results showed that the release time of the drug was 5-6 hours after taking the drug, and the release time was up to 9 hours.
Embodiment 3
[0063] Preparation of felodipine controlled-release preparation
[0064] Prescription (per 1000 tablets):
[0065] Tablet core: Felodipine 5g, HPMC K100LV 48.75g, sodium chloride 13g, crospovidone 3.25g, silicon dioxide 0.7g, medicinal ethanol 40g;
[0066] Coating layer: HPMC K100LV 180g, lactose 270g, magnesium stearate 2.25g, silicon dioxide 4.5g, medicinal ethanol 305g.
[0067] Preparation method: as described under Example 1.
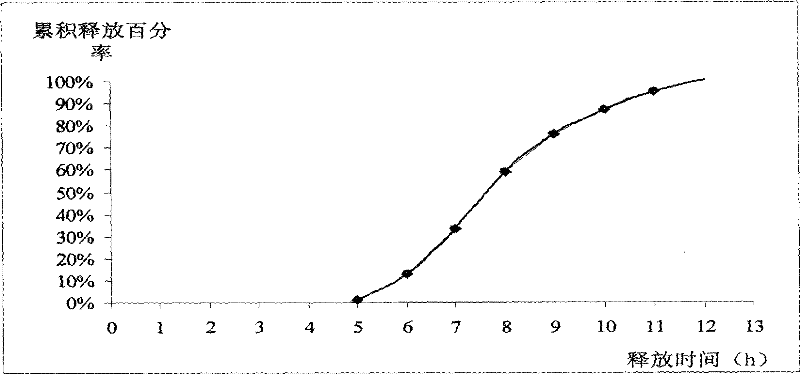
[0068] In vitro release assay: as described under Example 1. The experimental data are shown in Table 3 and attached image 3 .
[0069] The results showed that the release time of the drug was 5-6 hours after taking the drug, and the release time was 12 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com