Controlled release preparation of captopril and its preparation process
A technology of controlled-release preparations and preparations, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., which can solve the problems of long duration and achieve the effect of eliminating missed doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the preparation of captopril controlled-release preparation
[0048] Prescription (per 1000 tablets):
[0049] Tablet core: Captopril 25g, starch 2.5g, HPMC K4M 22.5g, PVP K30 1g, magnesium stearate 0.5g, micropowder silica gel 0.25g;
[0050] Coating layer: mannitol 295g, HPMC K4M 85g, PVP K30 14g, magnesium stearate 4g, micropowder silica gel 2g.
[0051] Preparation:
[0052] (1) The tablet core layer is separately granulated, and 8 g of medicinal ethanol is added during the granulation process, and the tablet is compressed (Φ5mm flat flush);
[0053] (2) The coating layer is granulated separately, and 75.4 g of medicinal ethanol is added during the granulation process;
[0054] (3) Press-coated chips (Φ12mm shallow concave punch): firstly fill each die hole with 200mg of coating particles, then place the tablet core in the center of the die hole, add 200mg of coating particles, and press to form a coated chip.
Embodiment 2
[0058] Prescription (per 1000 tablets):
[0059] Tablet core: Captopril 25g, starch 2.5g, HPMC K100M 22.5g, PVP K30 1g, magnesium stearate 0.5g, SiO 2 0.25g;
[0060] Coating layer: mannitol 285g, HPMC K100M 95g, PVP K30 14g, magnesium stearate 4g, SiO 2 2g. Preparation:
[0061] (1) The core layer of the tablet is granulated separately, and 7.8 g of medicinal ethanol is added during the granulation process, and the tablet is compressed (Φ5mm flat flush);
[0062] (2) The coating layer is granulated separately, and 54 g of medicinal ethanol is added during the granulation process;
[0063] (3) Press-coated chips (Φ12mm shallow concave punch): firstly fill each die hole with 200mg of coating particles, then place the tablet core in the center of the die hole, add 200mg of coating particles, and press to form a coated chip.
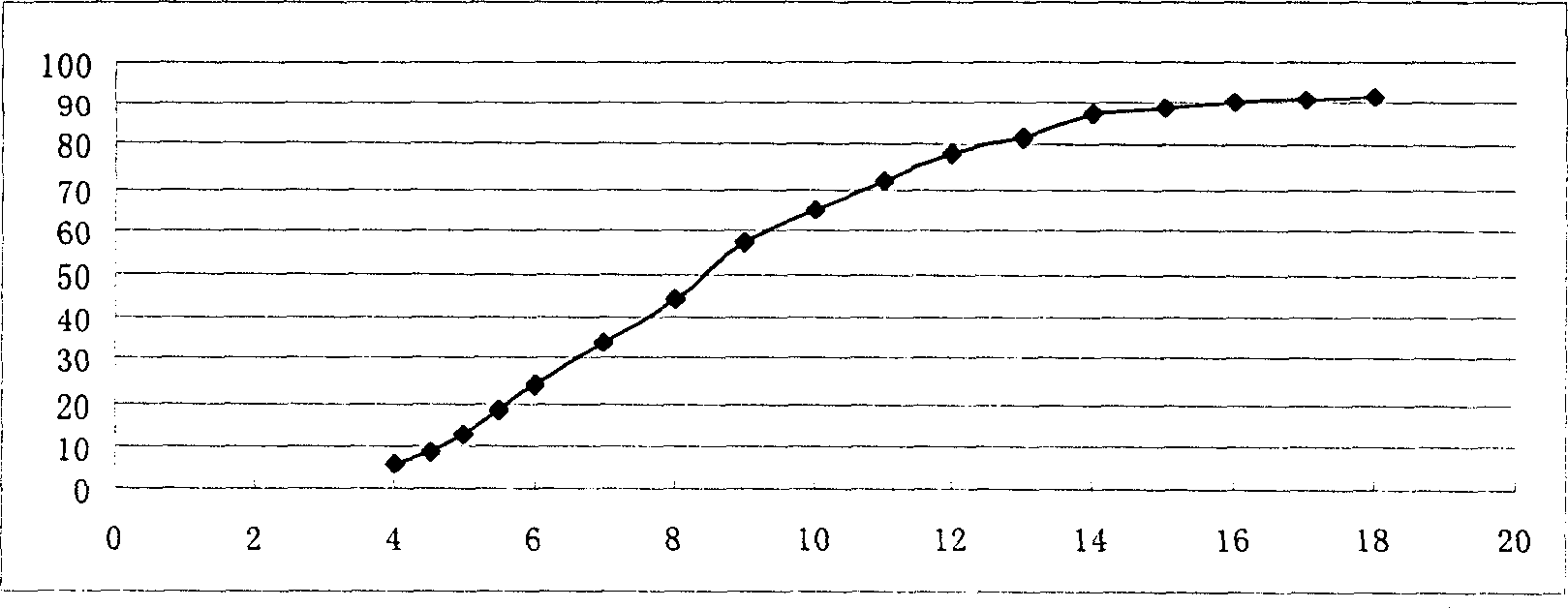
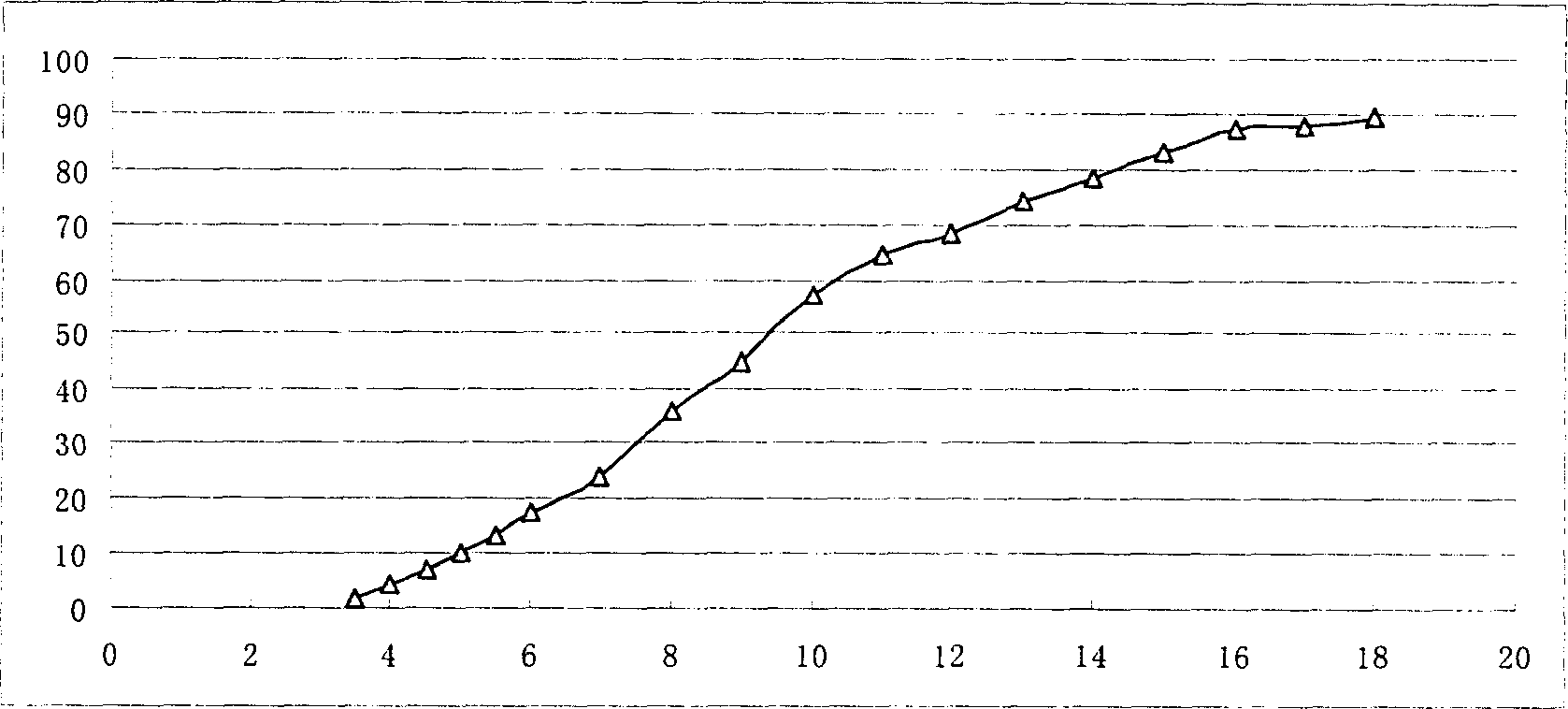
[0064] Determination of in vitro release: according to "Chinese Pharmacopoeia" 2000 edition two appendix XD first method adopts the device (basket m...
Embodiment 10
[0068] Embodiment 10: the preparation of captopril controlled-release preparation
[0069] Prescription (per 1000 tablets):
[0070] Tablet core: Captopril 25g, dextrin 14.25g, HPMC K100M 10.75g, PVP K30 1g, stearic acid 0.5g, micropowder silica gel 0.25g;
[0071] Coating layer: dextran 290g, HPMC K100M 90g, PVP K3014g, stearic acid 4g, micropowder silica gel 2g.
[0072] Preparation:
[0073] (1) The tablet core layer is granulated separately, and 4g of pure water is added during the granulation process, and the tablet is pressed (Φ5mm flat flush);
[0074] (2) The coating layer is granulated separately, and 58 g of pure water is added during the granulation process;
[0075] (3) Press-coated chips (Φ12mm shallow concave punch): firstly fill each die hole with 200mg of coating particles, then place the tablet core in the center of the die hole, add 200mg of coating particles, and press to form a coated chip.
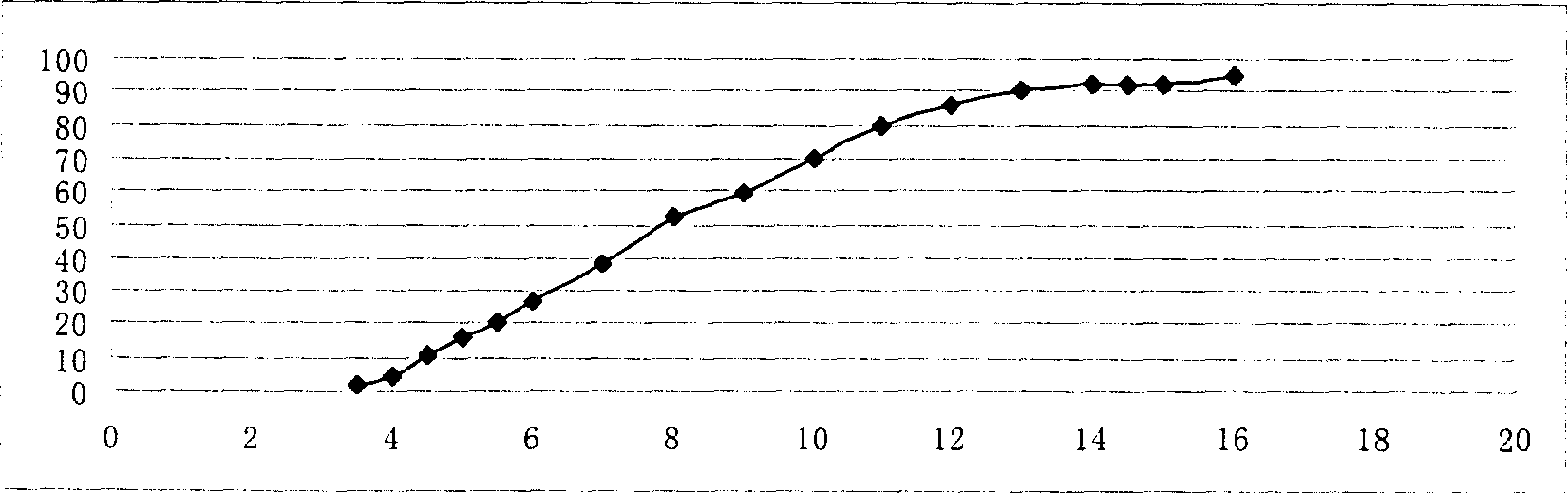
[0076] The in vitro release measurement method is the same as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com