Menthyl lactate process
A technology of menthyl lactate and lactic acid, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, organic chemistry, etc., can solve the problems of unrecorded yield and unprovided synthesis details, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
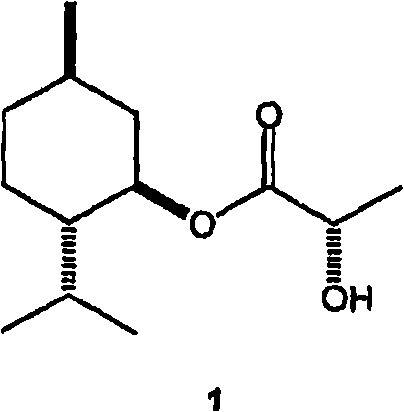
[0034] Preparation of 1-menthyl lactate by esterification and controlled hydrolysis
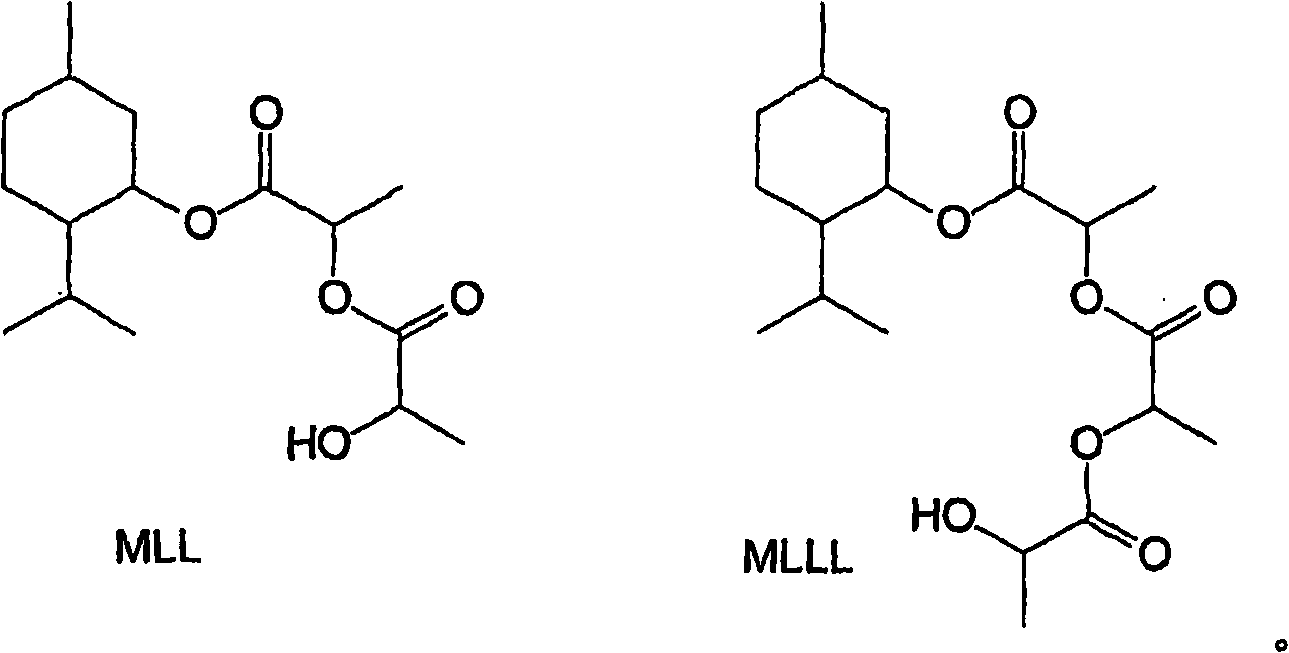
[0035] Esterification : Add 1-menthol (1440g), L-(+)-lactic acid (2880g, from Purac's HS-88 grade, 88% lactic acid in water) and heptane (720g). The stirred mixture was brought to reflux and water was periodically drained from the trap as it formed. After 32 hours and after 854 mL of the aqueous phase had been removed, the temperature of the mixture was gradually increased to 128°C. The mixture was cooled to ambient temperature and analyzed by gas-liquid chromatography (GC). The mixture contains: 5.4% unreacted menthol, 67.7% 1-menthyl L-lactate (ML), 0.6% lactide (cyclic dimer of lactic acid), 24.6% 1- Menthyl L-lactyl-L-lactate (MLL) and 0.4% 1-menthyl L-lactyl-L-lactyl-L-lactate (MLLL).
[0036] controlled hydrolysis : The esterified product was diluted with water (4230 g) and heptane (960 g). Aqueous sodium hydroxide solution (809 g, 50% NaOH) was then added dropwise over 30 minu...
Embodiment 2
[0042] Preparation of 1-menthyl lactate by sulfuric acid-catalyzed esterification and controlled hydrolysis
[0043] Esterification : The procedure of Example 1 was generally followed, using 1000 g 1-menthol, 1000 g L-(+)-lactic acid, 500 g heptane and 6 g concentrated sulfuric acid. After 2 hours and after 300 mL of the aqueous phase had been removed, the temperature of the mixture was gradually increased to 119°C. The mixture was cooled and analyzed by GC. The mixture contained: 6.4% unreacted menthol, 57.6% ML, 0.4% lactide, 32.2% MLL and 1.9% MLLL.
[0044] controlled hydrolysis : The esterified product was diluted with water (800 g) and heptane (500 mL). Aqueous sodium hydroxide solution (204 g, 50% NaOH) was then added dropwise over 70 minutes while stirring and cooling (cold water bath) the mixture so that the temperature did not exceed 30° C. and the pH did not exceed 13.1. Separate layers. The organic layer was diluted with water (1350 g) and treated with more...
Embodiment 3
[0051] Preparation of 1-Menthanyl Lactate by Esterification Catalyzed by Sodium Bisulfate
[0052] The esterification procedure of Example 1 was generally followed using 1000 g 1-menthol, 2000 g L-(+)-lactic acid, 500 g heptane and 10 g crystalline NaHSO 4 ·H 2 O. Reflux started at about 93°C and ended at about 123°C after about 581 g of water had been expelled (taking slightly less than 17 hours). Composition of the mixture (%, GC): Menthol 3.3%, ML 48.7%, Lactide 4.8%, MLL 36.5%, MLLL 5.6%. This mixture was then used in the hydrolysis experiments described below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com